Quantitative summary of QoL data confirms immune checkpoint inhibitor therapy is generally well-tolerated
ESMO Virtual Congress 2020 Sep 23, 2020
Dr. Brian D. Gonzalez of Moffitt Cancer Center in Tampa, USA presented findings from two meta-analyses at ESMO Virtual Congress 2020 which were designed to summarise quality of life (QoL) data to better educate patients receiving immune checkpoint inhibitor (ICI) therapy for cancer. These meta-analyses found that patients receiving ICI had better QoL than patients receiving non-ICI treatment. Dr Gonzalez reported findings on behalf of colleagues from this study, which was conducted with the aim of summarising QoL data to make QoL information more accessible to patients receiving ICI for cancer.
Patients treated with ICIs reported less change in QoL
The two meta-analyses were performed on publications of PD-1/PD-L1 and/or CTLA-4 inhibitors that provided mean-level QoL using the EORTC QLQ-C30 and/or EQ-5D questionnaires. One meta-analysis examined change in QoL in patients treated with ICIs from pre-treatment to follow-up, which occurred approximately 12 to 24 weeks later. The second meta-analysis compared QoL at follow-up between ICI and non-ICI regimens in randomised trials. Both had moderator analyses examining ICI regimen, comparator regimen, disease site, age, gender, follow-up period and risk of bias.
The investigators identified 20,323 publications, of which 26 studies met the inclusion criteria.
The first meta-analysis, which comprised 26 studies and 6,965 patients, indicated that QoL did not change over time in patients following ICI treatment (p > 0.05). Some subgroups reported improvements in QoL, such as patients treated with certain ICIs or for certain cancer types. Other significant moderators included sex and risk of bias (p values < 0.05).
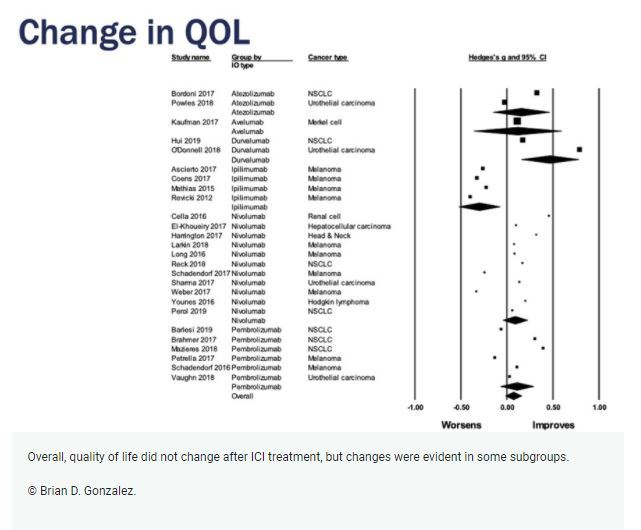
The second meta-analysis consisted of 16 studies and 6,536 patients; among these patients, 3,588 received ICI treatment and 2,948 patients received other, non-ICI therapy. Better follow-up QoL was observed in patients receiving ICI versus non-ICI regimens (p < 0.05). Significant moderators in this analysis included ICI regimen, cancer type, age and risk of bias (p values < 0.05).
Conclusions
The authors noted that their study is among the first to quantitatively summarise QoL in patients treated with ICIs.
These findings suggest that patients treated with ICIs have more stable and better QoL overall than patients treated with non-ICI regimens for similar cancer.
They concluded that these results confirm that, despite immune-related toxicities, ICIs are generally well-tolerated.
Funding for this study was reported from the US National Cancer Institute (grant K01 CA211789).
This article is a news release from ESMO 2020 Press Meeting. Read the original here.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries