Quality of life improvement greater with front-line pembrolizumab compared with chemotherapy in MCRC
ESMO Virtual Congress 2020 Sep 25, 2020
The improved progression-free survival (PFS) observed with single agent pembrolizumab versus standard of care chemotherapy in patients having previously untreated microsatellite instability-high (MSI-H) and/or deficient mismatch repair (dMMR) metastatic colorectal cancer (mCRC) in the phase III KEYNOTE-177 study was reflected by clinically meaningful improvements in health-related quality of life (HRQoL) that favoured pembrolizumab over chemotherapy, according to findings presented at ESMO Virtual Congress 2020 by Thierry André of the Hôpital Saint-Antoine in Paris, France.
In KEYNOTE-177 patients with confirmed MSI-H/dMMR mCRC who had not received prior systemic therapy for mCRC were randomly assigned 1:1 to receive pembrolizumab at 200 mg every 3 weeks for a maximum two years or investigator’s chemotherapy choice of mFOLFOX6 or FOLFIRI every two weeks, with and without bevacizumab or cetuximab.
The EORTC QLQ-C30, EORTC QLQ-CR29 and EQ-5D-3L questionnaires were administered at baseline and at various time points up to one year or end of treatment, whichever came first and at 30 days after treatment discontinuation.
This analysis contained data from 272 patients receiving ≥1 dose of study treatment and completing ≥1 HRQoL assessment. The change from least-squares mean (LSM) scores from baseline to prespecified week 18, 95% confidence interval (CI) and nominal 2-sided p values were calculated. Time to deterioration (TTD) was defined as a ≥10-point decline from baseline and was assessed by the Kaplan-Meier method and Cox regression model.
Patients receiving pembrolizumab showed improved global and self-reported health status as well as prolonged TTD in several parameters.
A total of 141 patients treated with pembrolizumab and 131 patients receiving chemotherapy were available for the HRQoL analyses. Compliance for all 3 questionnaires at baseline was 93% in both of the pembrolizumab and chemotherapy arms and compliance at week 18 was 88% versus 77% in the respective arms.
Evaluation of the LSM change from baseline to week 18 showed clinically meaningful improvement favouring pembrolizumab in QLQ-C30 global health status (GHS)/QoL and the EQ-5D VAS scales, which reflects the patient's self-rated health. The LSM difference in (GHS)/QoL was 9 (95% CI, 4.2-13.7; p = 0.0002) and the LSM difference in EQ-5D VAS scores was 7.4 (95% CI, 2.8-11.9; p = 0.0016) for patients receiving pembrolizumab versus chemotherapy.
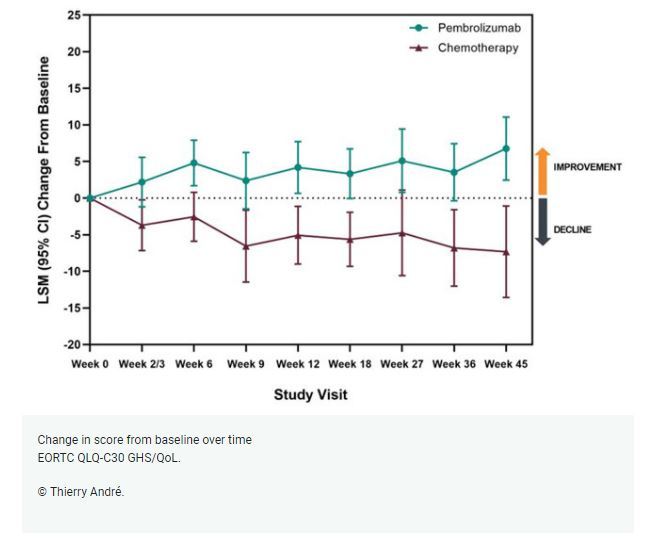
The TTD also favoured patients on pembrolizumab versus chemotherapy for GHS/QoL (hazard ratio [HR] 0.61; 95% CI, 0.38-0.98; p = 0.0195), physical functioning (HR 0.50; 95% CI, 0.32-0.81; p = 0.0016), social functioning (HR 0.53; 95% CI, 0.32 - 0.87; p = 0.0050), and fatigue (HR 0.48; 95% CI, 0.33 - 0.69; p ≤ 0.0001).
Conclusions
In the KEYNOTE-177 study patients receiving pembrolizumab monotherapy for previously untreated MSI-H/dMMR metastatic colorectal cancer demonstrated clinically meaningful improvements in HRQoL compared with similar patients treated with chemotherapy, according to the study authors.
This study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
This article is a news release from ESMO 2020 Press Meeting. Read the original here.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries