WHO warns India about bogus COVID vaccine
M3 India Newsdesk Sep 01, 2021
WHO has stated that counterfeit copies of Covishield have been in circulation in India and a few other countries, posing a huge health risk to the public. While the general public turns to Covishield for active immunisation, the falsified vaccine requires the healthcare providers to be extra vigilant.
For our comprehensive coverage and latest updates on COVID-19 click here.
Introduction
According to the World Health Organisation (WHO), counterfeit copies of India's main COVID vaccine, Covishield, have been discovered. Between July and August, authorities in India and Africa confiscated the dosages, according to a WHO statement.
Additionally, it said that the vaccine's manufacturer, Serum Institute of India, acknowledged the dosages were forged. The WHO has warned that counterfeit vaccinations saying they 'represent a grave threat to global public health'. It urged their abolition from circulation.
“Falsified Covishield was discovered at the patient level in Kolkata, India. This translates into medicinal goods being accessible, supplied, distributed, or given directly to patients," according to WHO spokesman Tarik Jaarevi. Several individuals have already been detained in Mumbai, Delhi, and Kolkata on suspicion of providing counterfeit shots. The rising number of bogus vaccination warnings demonstrates the problem's scope and is becoming a source of concern for health officials.
This WHO Medical Product Alert is in response to the discovery of counterfeit Covishield (ChAdOx1 nCoV-19 Corona Virus Vaccines (Recombinant) in the WHO African and WHO South-East Asia Regions. The WHO was notified of the fraudulent goods in July and August 2021.
Covishield's legitimate manufacturer (Serum Institute of India Pvt. Ltd.) has verified that the goods mentioned in this warning are forged. In Uganda and India, these fabricated goods have been reported at the patient level.
Genuine Covishield vaccine is recommended for active immunisation of people aged 18 years or older against coronavirus illness caused by the SARS-CoV-2 virus. Genuine COVID-19 vaccinations should be used in line with approved national regulatory advice.
COVID-19 vaccinations that have been tampered which represent a significant threat to global public health and impose an extra burden on vulnerable people and health systems. It is critical to identify and remove these forged goods from circulation in order to avoid causing damage to people.
The items listed in this warning have been verified as counterfeit due to the deliberate/fraudulent misrepresentation of their identity, composition, or source:
- Batch 4121Z040 - the expiration date on this product (10.08.2021) is forged
- Covishield 2ml - the authentic manufacturer does not make Covishield in a 2ml container (4 doses)
Consultation with regulatory agencies and the general public
WHO calls for enhanced vigilance across the supply chains of nations and areas that are likely to be impacted by these forged goods. Hospitals, clinics, health centres, wholesalers, distributors, pharmacies, and any other providers of medicinal goods should exercise more attention. All medical items must be purchased from licensed/authorised sources. The legitimacy and physical quality of the goods should be thoroughly inspected. In case of uncertainty, get guidance from a healthcare expert.
Products subject of WHO Medical Product Alert N°5/2021
| Product name | Covisield ChAdOx1 nCoV-19 Coronavirus vaccine (Recombinant) | |
| Stated manufacturer | Serum Institute of India Pvt. Ltd. | |
| Stated dose | 5 ml (10 doses) | 2 ml (4 doses) |
| Batch | 4121Z040 | Not stated |
| Manufacturing date | Not stated | Not stated |
| Expiry date | 10.08.2021 | Not stated |
| Packaging language | English | English |
| Identified in | Uganda | India |
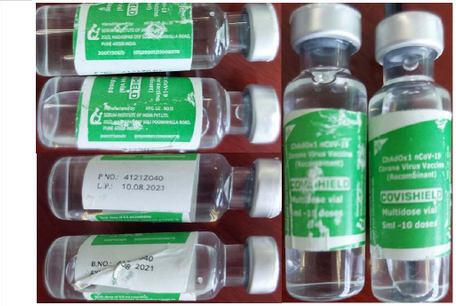
Falsified Covishield batch 4121Z040

Falsified Covishield, 2ml (4 doses)
Please do not utilise any of the above-mentioned items if you are in possession of them. If you have used these goods or have had an adverse reaction/event as a result of using them, you should seek urgent medical assistance from a competent healthcare practitioner and report the occurrence to the National Regulatory Authorities/National Pharmacovigilance Center.
National regulatory/health authorities are urged to inform WHO promptly if they find these tainted goods in their jurisdiction. Please email rapidalert@who.int if you have any information on the manufacturing, distribution, or supply of these goods.
Click here to see references
Disclaimer- The views and opinions expressed in this article are those of the author's and do not necessarily reflect the official policy or position of M3 India.
The author is a practising super specialist from New Delhi.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries