The Role of Gut Microbiota in Diabetes
M3 India Newsdesk Jul 18, 2024
This article explores the functions & metabolic actions of gut microbiota, emphasising its role in health & disease, particularly obesity & diabetes. It discusses the therapeutic potential of manipulating gut microbiota through medications, probiotics, and FMT to improve metabolic health & manage DM.
Functions of Gut Microbiota
The GM is involved:
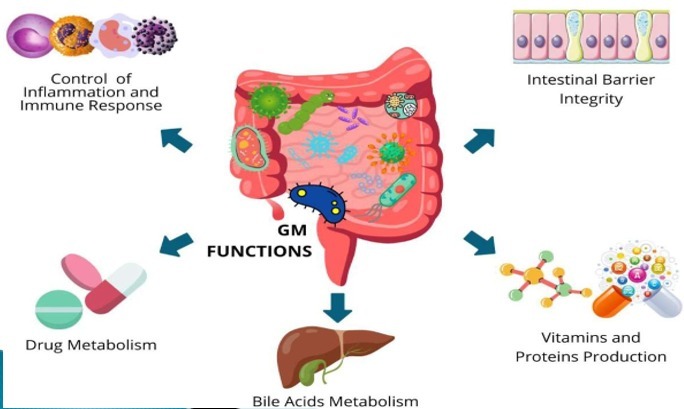
- The degradation of proteins and the synthesis of secondary bile acids (BAs)
- The degradation of xenobiotics
- Production of water-soluble vitamins
Also, it is gaining relevance not only for its crucial role in the development and maintenance of proper functioning of innate and adaptive immunity and gut-associated lymphoid tissue (GALT) but also for its implication in the process of energy extraction from otherwise indigestible foods.
The metabolic actions of gut microbiota
The number of bacteria inhabiting the human body to 3⋅8⋅1013, a number of the same order as human cells.
- Firmicutes (gram-positive) 60-80%
- Bacteroidetes(gram-negative),20-30%
comprises the entire genome, in addition to Actinobacteria and Proteobacteria.
A high number of bacterial species in the microbiota is a protective factor against metabolic diseases such as obesity, metabolic syndrome (MetS), and type-2 diabetes (T2D).
Additionally, compared to those who exhibit a higher number of genes, obese people who have fewer microbial diversity—and hence a lower metagenomic makeup—are more susceptible to weight gain, adiposity, insulin resistance, and inflammation.
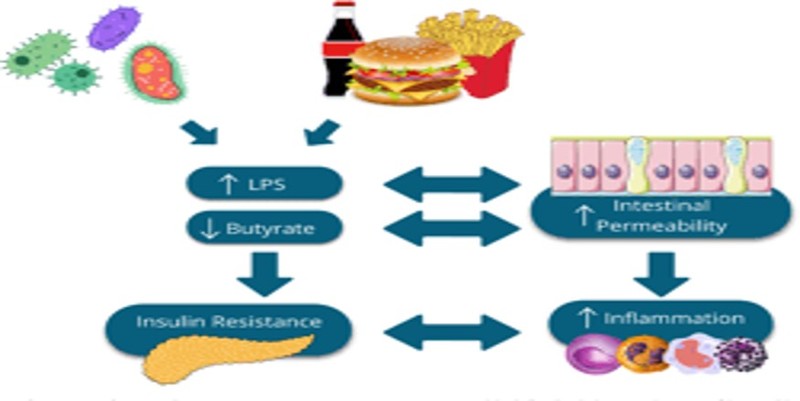
GM makes possible intestinal plant fibre fermentation, leading to the production of short-chain fatty acids (SCFAs), such as butyrate, propionate, and acetate. SCFAS are peculiar metabolites involved in the physiological activity of colonic cells and the regulation of appetite, insulin response, and inflammatory processes. Impaired SCFA levels are involved in the pathogenesis of metabolic, cardiovascular and oncological diseases.
Propionate and butyrate exert an anti-obesogenic action stimulating leptin and anorexigenic hormone synthesis while acetate predominantly presents obesogenic properties inducing ghrelin secretion and promoting fat storage.
Emerging evidence from experimental models and humans points to a reduction in butyrate-producing species, such as Faecalibacterium prausnitziiand Roseburia intestinalis, as one of the most important microbiota-related features responsible for the onset and development of T2DM.
Furthermore, the experimental model treated with tributyrin, a butyrate precursor drug, displayed protection from obesity, insulin resistance, and liver steatosis. Interestingly, insulin resistance is gradually developed in obese subjects receiving vancomycin, an antibiotic that inhibits the growth of butyrate makers.
However, propionate was found to impair insulin signalling in the experimental model and to elevate norepinephrine, glucagon, and FABP4 plasma levels postprandially in humans, hence causing insulin resistance. These findings were reported in a study involving the experimental model.
How gut microbiota is changed in Diabetes Mellitus and obesity?
Gut Microbiota in obesity and diabetes
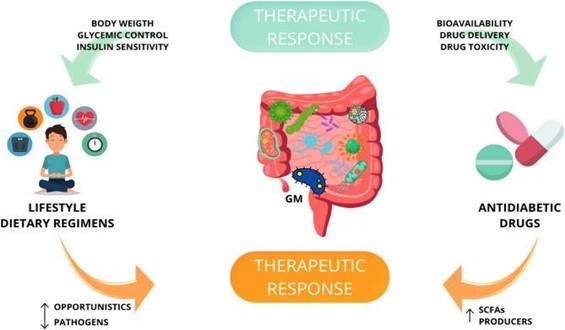
- THE CLINICAL TRIALS showed that probiotic supplementation decreases insulin resistance by increasing pathways of glucose metabolism and insulin sensitivity with beneficial effects on glycaemic control, HDL cholesterol, total-/HDL-cholesterol ratio biomarkers of inflammation and oxidative stress in diabetic patients.
- When one considers that low HDL cholesterol in fatty liver and diabetes is potentially a potential prognostic sign of hepatocellular carcinoma, these results are highly intriguing.
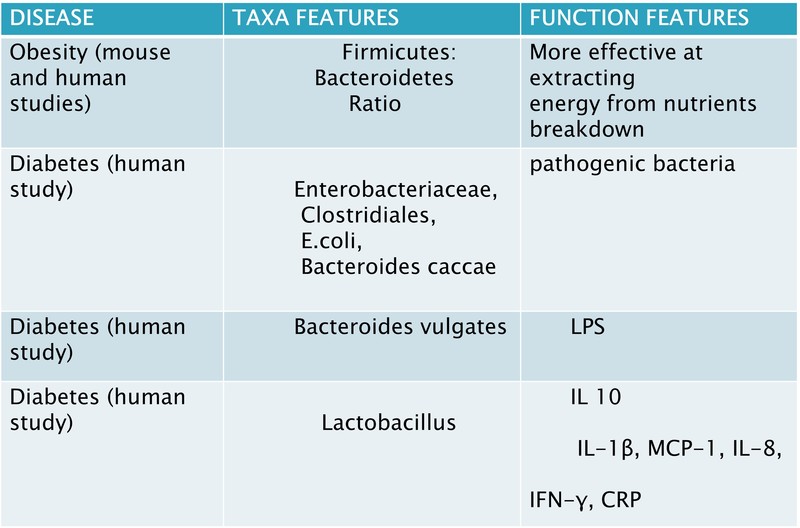
- Although the Bacteroidetes to Firmicutes ratio has been a valid measure of dysbiosis status a reduced ratio has been described in obese and metabolic subjects, its increase has instead been positively correlated with reduced glucidic tolerance.
Gut microbiota in obesity and diabetes
- Gram-negative bacteria that are harmful and opportunistic are more common in the GM of diabetic patients than commensal ones.
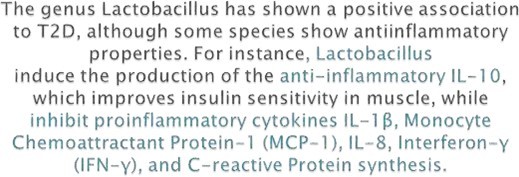
- The microbiota of diabetic patients has been discovered to include a higher number of pathogenic bacteria, including Prevotella copri and Bacteroides vulgates, as well as Enterobacteriaceae, different Clostridiales, Escherichia coli, Bacteroides caccae, and Lactobacilli.
- Proteobacteria are strongly pro-inflammatory, while Bacteroidetes are gram-negative bacteria whose presence is correlated with increased LPS. Conversely, their decline is linked to lower metabolic endotoxemia and reduced inflammatory status. It is well-known that this subclinical pro-inflammatory status due to LPSdependent production of inflammatory cytokines, such as Interleukin-1 (IL-1), IL-6, and Tumour Necrosis Factor-α (TNF-α), drives the development of insulin resistance and T2DM.
What are the interactions between anti-diabetic medicines and gut microbiota?
1. Gut microbiota and metformin
Metformin is the most studied antidiabetic oral drug. Given the diminished variety of antidiabetic effects attained by its intravenous administration, it is now clearly recognised that some of its therapeutic benefits are mediated by the GM.
It has been demonstrated that metformin-induced decreased abundance of Clostridium bartlettii, which is known to have a negative connection with indicators of insulin resistance, exists. Forty On the other hand, metformin-treated naïve T2D patients have an enrichment of Parabacteroides distasonis.
Metformin has also been associated with the strengthening of tight junctions. It has also been shown that the metformin-associated microbiota is more similar to healthy patients than untreated diabetics & there are indeed specific microbiotic clusters able to predict the efficacy of metformin therapy in diabetic patients:
- An increased presence of Prevotella copri appears to limit the ability to reduce glycated haemoglobin (HbA1c). Similarly, metformin-associated side effects are predicted by an increased level of Streptococcus parasanguinis prior to beginning antidiabetic medication.
- Since this increase is associated with the use of proton pump inhibitors and anti-platelet therapy, data on the GM composition could also be key to understanding how therapeutic failure or enhancement occurs in patients on polypharmacological treatments.
Metformin itself can strengthen the intestinal barrier and decrease LPS translocation by enhancing the intestinal expression of TJ proteins, indicating that metformin indirectly ameliorates insulin resistance also by modulating GM composition, SCFAs levels, improving intestinal barrier integrity.
Interestingly, FXR activation by the new ligand TC-100 in a murine model of obstructed BA flow prevents damage to the intestinal mucosa and is linked to an enrichment of Akkermansia muciniphila.
This suggests once more that intestinal barrier preservation plays a more general metabolic role in controlling intestinal inflammation, which may be extended to the management of chronic low-grade systemic inflammation, adiposopathy, and, ultimately, glucose tolerance.
2. Gut microbiota & glucosidase inhibitors
- Alpha-glucosidase inhibitors reduce postprandial hyperglycaemia inhibiting carbohydrate hydrolysis by binding to human intestinal maltase-glucoamylase and sucrase-isomaltase and may have beneficial effects on glycaemic control via GM. Do et al. showed that the injection of voglibose reduced the ratio of Firmicutes to Bacteroidetes, improving lipid and blood glucose metabolism?
- The administration of acarbose induced a microbial shift that increased the concentration of SCFAs and the experimental model's longevity. Acarbose-treated pre-diabetic patients showed reductions in Butyricicoccus, Phascolarctobacterium, and Ruminococcus and up-regulation of Lactobacillus, Faecalibacterium, and Dialister. Moreover, acarbose therapy increased Bifidobacterium longum presence and decreased LPS levels in T2D patients.
- SGLT-2 inhibitors lower plasma glucose by increasing the excretion of glucose in the urine. Numerous investigations found that SGLT-2 inhibitors had no effect on the composition of GM. Conversely, in a diabetic experimental model, Lee et al. showed that dapagliflozin treatment reduced Firmicutes to Bacteroidetes ratio and Oscillospira while increasing Akkermansia muciniphila. To fully understand how SGLT-2 inhibitors affect GM, more research is necessary.
3. Gut microbiota and GLP1 agonist
- Studies on the model have shown that GM controls satiety and glucose balance through the incretin hormone GLP-1, which is released by intestinal L cells. Accordingly, a type of antidiabetic medication called GLP-1 receptor agonists can alter the Firmicutes to Bacteroidetes ratio, changing the composition of GM.
- In the experimental model studies, liraglutide administration promoted the expression of SCFAs-producing bacteria such as Bacteroides, Lachnospiraceae and Bifidobacterium. Moreover, liraglutide administration causes Proteobacteria to decrease and Akkermansia muciniphila to rise.
4. Gut microbiota, gliptins & insulin sensitisers
- Pioglitazone, a thiazolidinedione, as well as DPP-4 inhibitors, such as sitagliptin and vildagliptin, also show the ability to modulate GM.DPP-4 inhibitors reduce blood glucose blocking the degradation of GLP-1 and they restore the GM composition increasing the abundance of Bacteroidetes.
- Sitagliptin treatment raised the expression of Firmicutes and Tenericutes in the experimental model, but vildagliptin treatment decreased the ratio of Firmicutes to Bacteroidetes and increased Lactobacilli spp.
What is FMT or stool transplantation and its role in the management of diabetes: Future aspects
Diabetes and faecal microbiota transplantation (FMT)
A strategy to harness the microbiota as an adjuvant strategy in the treatment of diabetes is FMT, also known as stool transplantation:
- The introduction of stool from a healthy donor into the digestive system of a recipient alters their GM to improve their health.
- For instance, FMT is currently one of the most successful therapies for recurrent and refractory Clostridium Difficile Infection (CDI), even in immunosuppressed or with underlying comorbidities patients.
How FMT is helping in diabetes?
- Emerging evidence is consistently showing that FMT may not only improve insulin sensitivity but also alter the natural course of type I diabetes by modulating autoimmunity.
- Plenty of preclinical data have been published in the last years and, despite model-related differences, have consistently shown the advantage of FMT in the improvement of insulin resistance, weight gain, cardio metabolism, and liver steatosis.
- A recent study compared the effects of FMT on donors who underwent bariatric surgery and donors with MetS.
- Recipients from bariatric patients showed a decrease in inflammatory indicators. A decrease in insulin sensitivity and an increase in secondary BAs were displayed in those transplanted from metabolic patients.
- A study published in 2019 (NCT01765517) has shown the benefit of a multi-strain probiotic supplementation over 6 months as monotherapy in decreasing HOMA-IR in T2D patients.
- Another study (NCT03100162) has shown the beneficial dose-dependent effects of a lyophilised powder containing live multispecies probiotic bacteria on cardiometabolic parameters and gut permeability of obese post-menopausal women.
- As demonstrated by other studies showing only a modest effect of GM manipulation on insulin resistance in T2D, it is clear now that the success of microbial modulation depends on the tested strains, their composition and diversity, the patient's pre-existing microbial diversity and his genetic fingerprint. However, there are certain hazards associated with FMT that need to be considered.
- The primary reasons for concern are the spread of infectious diseases or the establishment of dysbiotic conditions, which could accelerate the onset of issues related to genetic alteration. Furthermore, two cases of peripheral neuropathy in 2016 have been connected to FMT.
- FMT may not only improve insulin sensitivity but also alter the natural course of type I diabetes by modulating autoimmunity. Plenty of preclinical data have been published in the last years and, despite model-related differences, have consistently shown the advantage of FMT in the improvement of insulin resistance, weight gain, cardio metabolism, and liver steatosis.
Conclusion
- We discussed the potential of GM manipulation in diabetes focusing on the comprehension of the metabolic action of GM. The role of intestinal ecology in metabolic syndrome has been recently postulated for the connection between GM with inflammation, hyperinsulinemia, metabolic-associated fatty liver disease (MAFLD) and diabetes.
- There have been several discoveries on GM and gut–living axis modulation in diabetes. Probiotic administration's therapeutic efficacy in treating diabetes and associated consequences is now being studied in several experiments.
- We pointed to GM as a novel prognostic biomarker in diabetes and proposed the need for further future studies to depict a final scenario for the putative therapeutic role of prebiotics and probiotics modulate GM in diabetes and MAFLD.
Disclaimer- The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of M3 India.
About the author of this article: Dr. Kirti Benal is Divisional Medical Officer In Railway Hospital, North West Railways, Udaipur
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries