Seizure Classification and Pathophysiology
M3 India Newsdesk May 22, 2024
Seizures are classified based on onset and awareness levels, with focal and generalised types delineated, along with an 'unknown onset' category. This article explores epileptogenesis, neurotransmitter dynamics, and genetic influences in epilepsy pathophysiology.
Classification of seizures
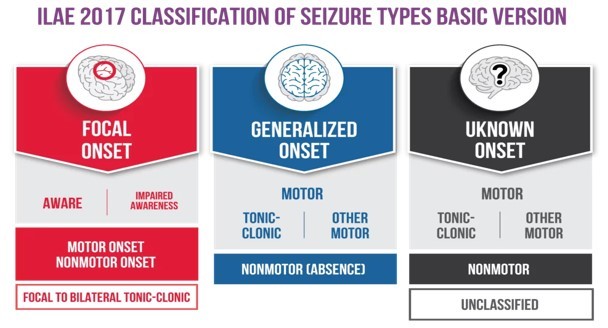
The classification of seizures was revised in 2017 by the International League Against Epilepsy (ILAE) [1], making some useful changes to the previous classification. A step-by-step approach is now implemented for completely defining the seizure type and activity.
The first step is to define the onset of seizures. The classification divides seizures into those with focal onset, meaning involving circuits (networks) in one hemisphere or side of the brain versus those that engage networks in both sides of the brain at the onset (generalised onset). If the onset is unknown, the seizure falls into the unknown onset category. Later, the seizure type can be changed if the onset of a person’s seizures becomes clear.
If the seizure is of focal onset, the next step is to define the level of awareness in them. A seizure is classified as “focal aware” if awareness is intact, even if the person is unable to talk or respond during the seizure (previously classified as a “Simple Partial Seizure”). A seizure is classified as “focal impaired awareness” if awareness is impaired at any time during the seizure (previously described as “Complex Partial Seizure”). A seizure can start focally in the brain and spread to both sides of the brain, resulting in a “focal to bilateral tonic-clonic seizure.” This was previously called a “secondarily generalised tonic-clonic seizure.”
The third step is to describe motor and other symptoms in focal seizures. A focal motor seizure means some type of movement occurs during the event. For example, twitching, jerking, or stiffening movements of a body part or automatisms (automatic movements such as licking lips, rubbing hands, walking, or running). A focal non-motor seizure includes other symptoms such as changes in sensation, emotions, thinking, or experiences.
In case the seizure is of generalised onset, it is subclassified into motor or non-motor (absence). The generalised tonic-clonic seizure term is used to describe seizures with stiffening (tonic) and jerking (clonic). This corresponds to the previously classified “grand mal” seizures. The generalised absence of seizures corresponds to the old term “petit mal” seizures. These seizures involve brief changes in awareness and staring, and some may have automatic or repeated movements like lip-smacking.
Following this basic classification of seizure, there is an expanded classification proposed by ILAE. This expanded classification keeps the framework of the basic classification but adds more seizure types as subheadings. Since seizures often have several different symptoms and behavioural signs, the seizure is named for the first prominent symptom or sign.
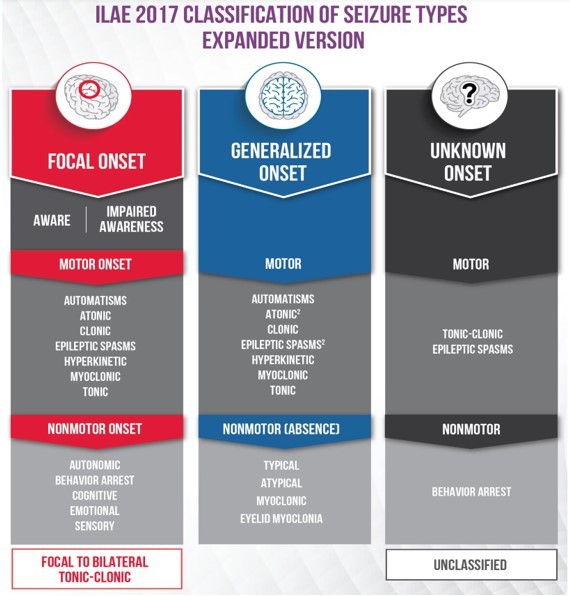
Following are the important changes which were made in the new classification compared to the previous one:
- Change of “partial” to “focal”.
- Focal to bilateral tonic-clonic seizure replaces secondarily generalised seizure.
- Seizures of unknown onset may have features that can still be classified.
- Awareness is used as a classifier of focal seizures.
- The terms dyscognitive, simple partial, complex partial, psychic, and secondarily generalised were eliminated.
- New focal seizure types include automatisms, autonomic, behaviour arrest, cognitive and emotional.
- New generalised seizure types include absence with eyelid myoclonia, myoclonic absence, myoclonic–tonic-clonic, myoclonic–atonic, and epileptic spasms.
- Atonic, clonic, epileptic spasms, myoclonic, and tonic seizures can be either focal or generalised.
Pathophysiology of epilepsy
Epilepsy is a complex neurological condition resulting from abnormal, excessive, and synchronous firing of neuron groups within the brain. These abnormal networks can occur because of structural, infectious, or metabolic insults [2].
The term “epileptogenesis” refers to the sequence of events that transforms the brain from a normal state to one predisposed to seizures. It makes the neuronal tissue hyperexcitable, hence making them capable of generating spontaneous seizures and further leading to the development of epilepsy.
The progression of epilepsy is characterised by neuroinflammation, along with structural and molecular changes in the brain. Microglia tend to play an important role in neuroinflammation and axonal sprouting [3].
After the occurrence of seizures, microglia are activated in the brain where they act as local macrophages, keeping homeostasis in check and responding to injury. After seizures, cytokines such as IL-1β, IL-6, and TNF-α are released. These cytokines influence NMDA receptors, synaptic plasticity, GABAergic neurotransmission, and neuronal excitability, hence contributing to the development and recurrence of seizures [4].
Another theory which tends to explain the phenomenon of epileptogenesis is the imbalance between excitation and inhibition. The imbalance occurs due to an increase in extracellular Glutamate concentrations and/or a decrease in GABA concentration, resulting in excitotoxicity, convulsions, and cell death [5].
Glutamate is a crucial neurotransmitter of the brain; it has an important role in various cognitive processes and acts in the extracellular space. It is released by glutaminergic neurons and acts on the ionotropic and metabotropic receptors. In the astrocytes, glutamate is converted to glutamine, transported back to the neurons, and reverted to glutamate for subsequent release. Hence, a balance between the neurons and astrocytes is crucial for maintaining an optimal glutamate concentration [6].
By converting glutamine into glutamate and then gamma-aminobutyric acid (GABA) by the activity of glutamate decarboxylase, astrocytes also enhance the glutamine supply to GABAergic neurons. The formed GABA is then packaged into vesicles for release. GABA is the main inhibitory neurotransmitter in the CNS; deficiency or absence of GABA leads to overexcitation of neurons subsequently causing seizures [7].
Besides neurotransmitters, cases of severe epilepsy have been found associated with genetic factors [8]. The main influence is related to mutations in the ion channel neurotransmitters; however other mechanisms like mutations in transcription factors, intracellular signalling molecules, metabolic enzymes, and even genes in the mitochondrial complex have been discovered in individuals with genetic epilepsy.
Some common genetic mutations which have been associated with genetic epilepsy are related to CACNA1E, GABRB3, GRIN2A, GRIN2B, KCNA2, DEPDC5, etc.
To conclude, epilepsy is a complex neurological disorder with multiple mechanisms, risk factors and genetic influences. A better understanding of these mechanisms will help develop targeted antiepileptic drugs in the future for better management of refractory cases of epilepsy.
Disclaimer- The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of M3 India.
About the author of this article: Dr. Annesh Bhattacharjee is a Consultant Neurologist, at Cosmo Medical, Guwahati.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries