Remineralising & Demineralising Agents: Introduction & Recent Advances
M3 India Newsdesk Feb 07, 2025
This article provides a comprehensive understanding of remineralisation science, the latest advancements in dental materials, & practical insights into choosing the best strategies for preventing & managing early tooth decay.
Remineralising Agents
- Dental caries is an infectious microbiologic disease of teeth that results in localised dissolution and destruction of calcified tissues.
- The process of caries formation is a cycle of remineralisation and demineralisation with various stages being either reversible or irreversible.
Demineralisation
Silverstone (1977) defined demineralisation as the process of removing minerals, in the form of mineral ions, from dental enamel.
Remineralisation
Silverstone (1977) defined remineralisation as the process whereby partially demineralised enamel is repaired through recrystallisation of tooth enamel mineral salts.
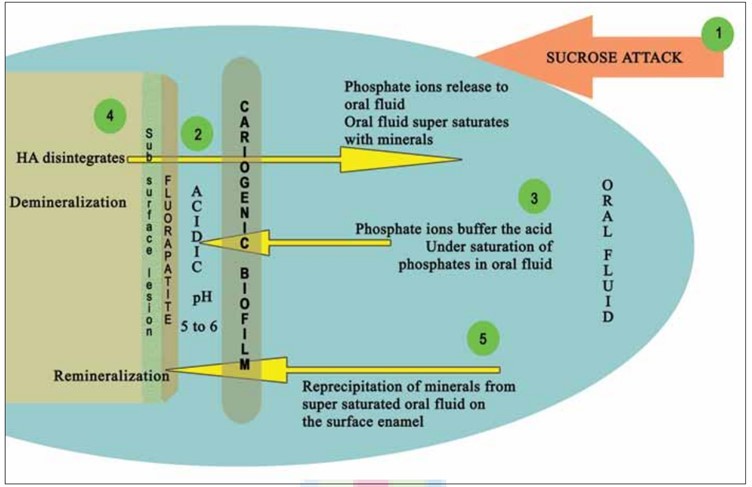
Demineralisation of Hydroxyapatite (HA) and Remineralisation with Fluorapatite (FA) - Depicted as Stages 1-5:
Stage 1: Fermentable sucrose intake.
Stage 2: Microbes in cariogenic plaque metabolise them releasing acid in the biofilm-tooth interface. The pH in the interface drops below the critical pH of HA.
Stage 3: Phosphate ions from oral fluid buffer acidic ions resulting in undersaturation.
Stage 4: HA disintegrates to release the phosphate ions back into the oral fluid till it supersaturates-Demineralisation.
Stage 5: Supersaturated oral fluid re-precipitates the minerals onto the disintegrated enamel. If fluoride also deposits FA is formed on the superficial layer - Remineralisation. Sub-surface demineralisation remains.
Enamel Demineralisation in the Presence of (F) Fluoride in Dental Biofilm:
Sugars (sucrose, glucose, fructose) are converted to acids in biofilm. When pH decreases to below 5.5, undersaturation happens concerning hydroxyapatite (HA) and then reaches biofilm fluid, resulting in mineral dissolution. However, if pH is higher than 4.5 and F is present, the biofilm fluid is supersaturated concerning fluorapatite (FA) and there is reprecipitation of minerals in the enamel. As a consequence, net demineralisation is reduced.
Requirements of a Remineralising Agent
- Should deliver calcium and phosphate into the sub-surface
- Should not deliver any excess calcium
- Should not favour calculus formation
- Should work at an acidic pH so as to stop demineralisation during a carious attack
- Should be able to work in xerostomic patients as saliva cannot effectively stop the carious process
- Should be able to boost the remineralising properties of saliva
- The novel materials should be able to show some benefits over fluoride
Indications
- An adjunct preventive therapy to reduce caries in high-risk patients
- Reduce dental erosion in patients with gastric reflux or other disorders
- To reduce decalcification in orthodontic patients
- To repair enamel in cases involving white-spot lesions
- Orthodontic decalcification or fluorosis or before and after teeth whitening and desensitising sensitive teeth
Remineralisation Systems
1. Natural remineralisation solely is inadequate
- The remineralisation capability of saliva is undeniable. It delivers tooth minerals in ionic form in their assimilability state to develop calcified tissues as well as their sustainability throughout life.
- At neutral pH, saliva is in a state of supersaturation with tooth mineral ions, thereby guaranteeing these minerals are available for mineral-deficient lesions.
- Nevertheless, long-term studies that assessed the progression of white spot lesions (WSL) observed a reduction in the size of WSL, but they mainly seemed to be unchanged even after two years.
- Hence, net salivary remineralisation is time-consuming.
2. Fluoride based remineralisation
- Since 1980’s customary fluoride-based remineralisation has been regarded as a benchmark for rendering carious lesions inactive. However, fluoride-mediated salivary remineralisation is limited distinctly to the external 30 µm of the tooth.
- Due to a small narrow “dose gap” between the benefit of caries reduction v / s side effects of fluoride, regulatory specialists have restricted the concentration of fluoride in toothpaste to approximately 1000 - 1500 ppm. However, in children < 6 years of age, the dose is even lower for early lesions’ remineralisation.
Requirement for Non-fluoride Strategies
- The effect of fluoride seems to be more extensive on smooth-surface caries, but it displays limited efficacy on pit and fissure caries.
- To avoid the potential for adverse effects (e.g., fluorosis) a high-fluoride strategy cannot be followed.
- Fluoride toxicity increases with inadequate nutrition.
- In spite of proving its remineralising efficacy, there is a wide gap when it comes to complete rehabilitation.
- The anti-fluoride lobby, which is staging pressure, poses certain legal implications when it comes to using fluoride.
- The availability of fluoride products is still questionable in certain nations.
Non-Fluoride Enamel Remineralising Systems
- Biomimetic Regeneration Systems
- Agents that deliver calcium and phosphate ions to tooth surface
- Agents that modify biofilm
- Agents that neutralise organic acids
- Antimicrobials and antibiotics
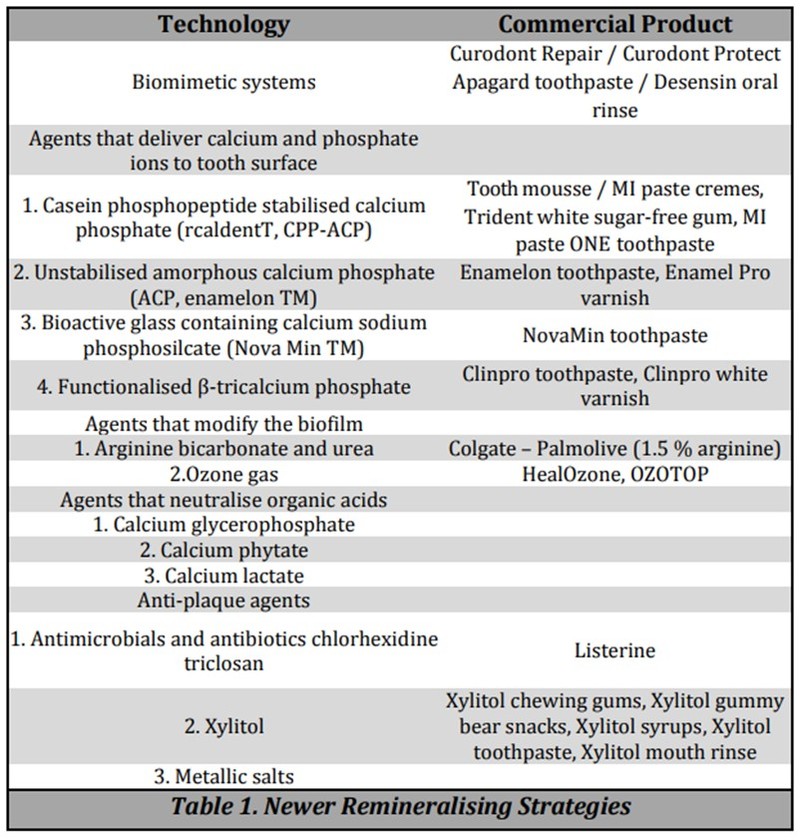
1. Casein Phosphopeptide- Amorphous Calcium Phosphate (CPP-ACP)
- Casein is a major protein group found in milk. This protein nanotechnology was developed by Eric Reynolds and co-workers.
- CPPs are produced from a tryptic digest of casein, aggregated with calcium phosphate and purified through ultrafiltration.
- Casein phosphopeptide forms nanoclusters with amorphous calcium phosphate thus providing a pool of calcium and phosphate which can maintain the supersaturation of saliva.
- Since CPP–ACP can stabilise calcium and phosphate in solution, it can also help in buffering of plaque pH so calcium and phosphate levels in plaque are increased.
- Therefore calcium and phosphate concentration within subsurface lesions is kept high which results in remineralisation. It is available as tooth crèmes, toothpaste chewing gums and as varnish along with fluoride.
2. Amorphous Calcium Phosphate (ACP)
- ACP technology- by Dr. Ming S. Tung in 1999.
- ACP was incorporated into toothpaste called Enamelon and later reintroduced in 2004 as Enamel Care toothpaste.
- It requires a two-phase delivery system to keep calcium and phosphorous components from reacting with each other before use.
- Current sources of calcium and phosphorous are two salts, calcium sulfate and dipotassium phosphate. When two salts are mixed, they rapidly form ACP that can precipitate onto the tooth surface. This precipitated ACP can then readily dissolve into saliva and can be available for tooth remineralisation.
3. Bioactive Materials
- A bioactive material is defined as a material that stimulates a beneficial response from the body, particularly bonding to host bone tissue and to the formation of a calcium phosphate layer on the material surface.
- Bioglass (BG) is a class of bioactive material which is composed of calcium, sodium, phosphate, and silicate. They are reactive when exposed to body fluids and deposit calcium phosphate on the surface of particles.
- In vitro and in vivo studies have shown that BG particles can be deposited onto dentine surfaces and subsequently occlude dentinal tubules by inducing the formation of carbonated HAP-like materials.
4. Tricalcium Phosphate (TCP) Clinpro Tooth Creme
- A bioactive formulation of tri-calcium phosphate and simple organic ingredients.
- It provides a barrier that prevents premature TCP–fluoride interactions and also facilitates targeted delivery of TCP when applied to teeth.
- Products available with TCP include 5000 ppm sodium fluoride dentifrice and 5% sodium fluoride varnish. Studies have concluded that TCP provided superior surface and sub-surface remineralisation compared with 5000 ppm fluoride and CPP-ACP.
5. Nano-Hydroxyapatite
- Nano-hydroxyapatite had the potential to remineralise initial enamel lesions.
- A concentration of 10% nanohydroxyapatite may be optimal for remineralisation of early enamel caries.
6. Bioactive Glass (Sodium Calcium Phosphosilicate)
- A biomimetic material, affects signalling pathways, thereby restoring tooth structure.
- NovaMin contains Bioactive glass and calcium sodium phosphosilicate.
- It has antimicrobial activity towards Streptococcus mutans and Streptococcus sanguis.
- It aids in remineralisation of tooth structure, especially in patients with systemic problems.
7. Xylitol
- A non-fermentable sugar alcohol that has shown anti-caries effect. It decreases Streptococcus mutans (MS) levels in plaque and saliva.
- Xylitol also encourages remineralisation by enhancing the flow of saliva as chewing gum or large xylitol lozenge resulting in a superior buffering potential against an acidic environment.
8. Cheese
- Cheese is a powerful sialogogue. It elevates levels of calcium, and/or possibly phosphorus, in dental plaque which might inhibit demineralisation through a common-ion effect, or might enhance remineralisation during periods of high pH.
- Rugg- Gunn et al (1975) demonstrated that eating cheese after a sugar-containing snack raised the pH of plaque back to a safe level.
9. Theobromine
- Theobromine, found in cocoa and chocolate exhibits crystalline growth in the enamel, making it less susceptible to acid attack.
- Upon comparative evaluation remineralising efficacy of dentifrices containing theobromine and sodium fluoride. It was observed that the former exhibited a significantly higher mineral gain than the latter.
10. Grape Seed Extract
- It inhibits the glucosyltransferase enzyme produced by S mutans resulting in the inhibition of dental caries.
- Grape seed extract is a potent substitute for fluorides for the prevention of root caries in elderly patients.
11. Ozone
- It has an immediate direct effect on carious lesions by eliminating the acid-producing bacteria and delaying the indirect effect by promoting the remineralisation of the lesions.
- Al-Duboni in 2013 conducted a study to evaluate the efficiency of ozone alone and with a remineralising solution and concluded that Ozone treatment either alone or combined with a remineralising solution is effective for remineralisation of initial fissure caries lesions.
12. Self-assembling Peptides
- P11 - 4 can cause a reversal of initial lesions on occlusal and proximal surfaces.
- P11 - 4 treated carious lesions showed superior aesthetics and higher radiopacity that remained stable even 6 - 12 months posttreatment.
13. Remineralisation Using Iontophoresis
- Iontophoresis is the process of depositing ionic drugs into tooth surfaces with low-voltage electric current.
- The term iontophoresis is simply defined as ion transfer (ionto = ion; phoresis = transfer). This method can able to achieve deeper penetration of drug ions into the desired target area than obtained with only topical application.
- Due to an application of current iontophoresis technique, remineralisation takes place at a faster rate in the incipient lesion as the main mechanism of action of fluoride is by the formation of calcium fluoride (CaF2) layer on the tooth surface which acts as a fluoride reservoir.
14. Lasers in Remineralisation
- It has been proposed that lasers can be used as an adjunct to conventional fluoride the remineralising the tooth structure.
- Hossain et al. reported that a combination of CO2 Laser with 2% NaF was more potent in preventing dental caries than CO2 laser irradiation alone.
Closing Remarks
In recent years, the focus of restorative dentistry has been directed toward a conservative approach, out of which remineralisation procedures are the most preferred and optimal way of regeneration of lost tooth structure.
Disclaimer- The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of M3 India.
About the author of this article: Dr Neha Kalantri is a practising dentist from Nashik.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries