Parasitic Infections Affecting the Nervous System: Know the Clinical Manifestations
M3 India Newsdesk Feb 23, 2024
Dive into the realm of parasitic infections affecting the nervous system, covering cases like cerebral malaria and neurocysticercosis. Gain insights into diagnostics and treatments, unravelling the impact on neurological health.
Parasitic infections are a common occurrence in developing countries and are an important cause of neurological morbidity. They invade the human nervous system through multiple mechanisms. Accessing blood circulation through the gut, inoculation by a vector bite, or directly through the olfactory mucosa.
In the central nervous system (CNS), they may remain asymptomatic, cause some acute manifestations, or develop into a chronic disease.
This article highlights the most common parasitic nervous system infections seen in routine clinical practice, along with salient features of some rare ones.
Cerebral malaria
Malaria is the most common cause of parasitic mortality and morbidity worldwide, affecting not only the inhabitants of tropical countries but also travellers. Out of the four species, only Plasmodium falciparum is seen to affect the CNS and cause severe disease.[1]
P. falciparum is transmitted through the bite of Anopheles species mosquito. The life cycle involves various stages involving the liver, RBCs in humans and the GI tract of mosquitoes.
Cerebral malaria refers to encephalopathy associated with P. falciparum infection in the absence of any other cause. It primarily affects children and immunocompromised adults. Brain injury results from hypoxia and ischaemia secondary to sequestration of infected RBCs in the cerebral blood vessels.[2]
Besides the usual systemic symptoms of malaria (fever, malaise, etc.), patients present with seizures (focal onset or generalised), behavioural abnormalities, and alteration of the sensorium, subsequently resulting in coma.
Adults have fewer complications and neurologic sequelae compared to children. Neurological symptoms can also occur from high fever, adverse effects of antimalarials, metabolic derangements and severe anaemia.
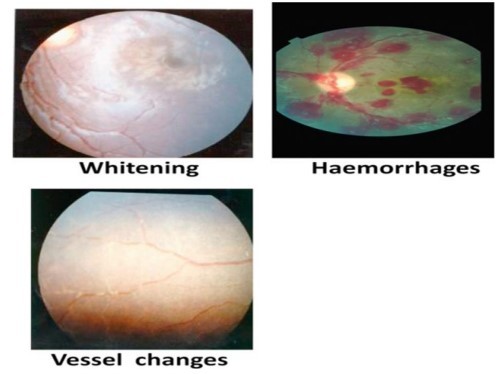
Fig. 1: Typical changes seen in Malarial Retinopathy.
The presence of retinopathy (Fig. 1) is both sensitive and specific for malaria and is associated with a poorer prognosis.[3] Neuroimaging (mainly MRI) may reveal the presence of focal infarcts, and cerebral and cerebellar oedema, while CSF study is largely normal.
Malaria treatment:
Initiation: Early use of antimalarials
- Intravenous Artesunate (I/V Artesunate)
Supportive measures:
- Control of seizures
- Other supportive interventions as needed
Neurocysticercosis
Neurocysticercosis (NCC) is caused by the larval stage of the pork tapeworm, Taenia solium, which is endemic in most developing countries. It is a major preventable cause of epilepsy in these countries.[4]
Humans acquire NCC by ingesting the tapeworm eggs via faecal/oral transmission.
Clinical manifestations
- Clinical manifestations of NCC depend on the location of the lesion, its size, number, and stage as well as the inflammatory response of the host.
- Seizures & headaches are the main features of intraparenchymal NCC, whereas the extraparenchymal (subarachnoid and intraventricular) forms present with intracranial hypertension, arachnoiditis and hydrocephalus.
- The various evolutive stages of NCC can be well visualised on neuroimaging. Initially, it begins with a viable cyst (Vesicular Stage) which later degenerates (Colloidal Stage) and is associated with inflammation.
- Subsequently, it transforms into an inflammatory nodule (Granulo-nodular Stage) and then disappears.
- In a few cases, it may reappear as a calcified scar (Calcified Stage). Sometimes, uncontrolled parasite growth in the subarachnoid space leads to the formation of large parasite clusters resembling a bunch of grapes (racemose neurocysticercosis) (Fig. 2)
- Hence, NCC has a long latent period in which the brain cysts evade the immune mechanisms of the host. It is the death of the cyst which brings about the inflammatory reaction and subsequent clinical features of NCC.
Recent guidelines recommend the usage of both CT and MRI for the diagnosis of NCC5. Detection of antibodies against T. solium can be done by the enzyme-linked immunoelectrotransfer blot (EITB) assay using lentil-lectin purified glycoprotein parasitic antigens in serum. Antigen detection confirms the presence of living parasites and helps in monitoring treatment.[6]
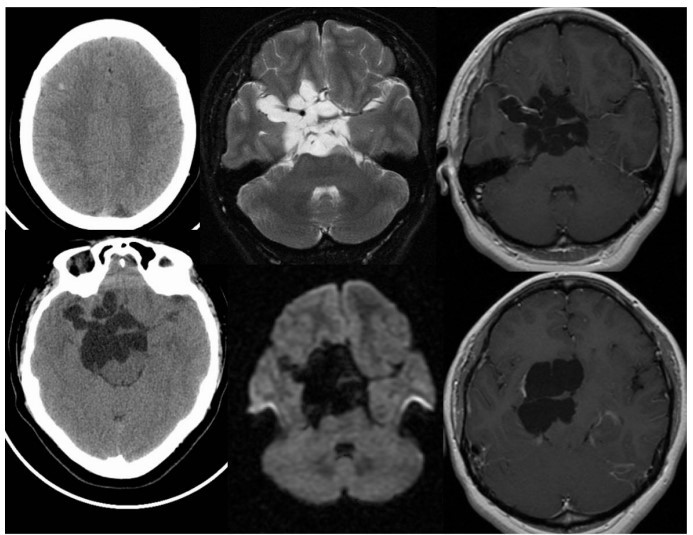
Fig 2: Racemose form of NCC. Multiple CT and MR images demonstrate multilobulated and multiseptated CSF signal/density structures within the suprasellar cistern, associated with localised mass effect and a few linear areas of peripheral enhancement.
Management of NCC:
- Therapy for NCC included management of seizures, raised intracranial pressure and the use of antiparasitic drugs (with/without corticosteroids).
- Both viable and degenerating cysts contain live parasitic tissue and antiparasitic drug usage is beneficial in both cases.
- However, the use of antiparasitic drugs may initially worsen neurological symptoms because of an increase in inflammation around the cyst.
| Albendazole treatment | |
| Single cyst | Multiple cysts |
| Dose: 15mg/kg/d | Combination therapy: Albendazole plus praziquantel |
| Duration: 7 to 15 days | Albendazole dose: 15mg/kg/d |
| Praziquantel dose: 50mg/kg/d | |
| Duration: 10 days | |
| Surgical intervention | |
| Indications | |
| 1. Hydrocephalus management | |
| 2. Removal of large intraventricular cysts | |
Toxoplasmosis
It is caused by the protozoan Toxoplasma gondii, which infects humans by:
- Consumption of infected meat containing tissue cysts
- Ingesting infective oocysts in soil
- Organ transplantation
- Blood transfusion or mother-to-foetus transplacental infection
It is a frequent cause of neurological disease in HIV-infected immunocompromised patients.
Congenital infection during pregnancy may lead to abnormalities and parenchymal brain tissue calcification. Retinal scars can be seen in early childhood infection.
In adults who are immunocompromised, tissue cysts may reactivate, resulting in clinical symptoms (fever, headache, focal neurological deficits, raised intracranial pressure, seizure, etc.)
Neuroimaging reveals the presence of ring-enhancing lesions mostly seen in the basal ganglia and subcortical white matter (Fig 3). The eccentric-target and concentric-target signs are highly specific for the diagnosis of CNS Toxoplasma infection.
Serology helps in confirming the diagnosis: a positive IgM test or rising IgG antibody titres are indicative of acute infection.[7]
Negative serology results may be found in HIV-infected patients. PCR of CSF is helpful in neurological disease.
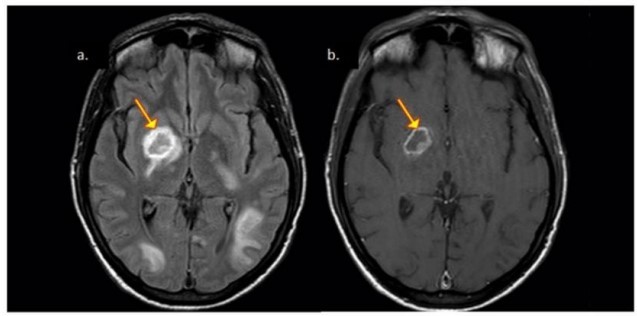
Fig 3: Patient with CNS toxoplasmosis. A. Axial FLAIR exhibiting hypointense lesion in the nuclei of the base with hyperintense peripheral halo (arrow). B. Axial T1 exhibiting the same hypointense lesion with peripheral ring enhancement. Mural nodule (eccentric target signal).
Treatment includes early initiation of a combination of pyrimethamine and sulfadiazine for 6 weeks or more with Leucovorin added to reduce haematological toxicity.
Patients with a sulfa allergy can be given-
- Clindamycin or
- Atovaquone
Alternatively, Trimethoprim-Sulfamethoxazole can be given resulting in a lower pill burden, less frequent dosing, and lower cost of therapy. Treatment efficacy is assessed by imaging follow-up. Non-responders should be evaluated for the presence of Primary CNS Lymphoma which is a close differential diagnosis.
Other parasitic infections of the central nervous system (CNS)
- Free-living amoebae:
Types: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri
Modes of invasion: Through the skin, respiratory tract, or directly through olfactory mucosae
Diseases caused:
- Naegleria: Primary amoebic meningoencephalitis
- Acanthamoeba and Balamuthia: Granulomatous amoebic encephalitis (more chronic)
| Trypanosomiasis | |
| Subdivisions | Caused by |
| Sleeping Sickness (African Trypanosomiasis) | T. brucei |
| Chagas Disease (American Trypanosomiasis) | T. cruzi |
- Insect vectors:
- Tsetse fly for Sleeping Sickness
- Bugs of the genus Triatoma for Chagas Disease
| Other rare parasitic human infections | |
| Infection | Caused by |
| Toxocariasis | Toxocara canis, Toxocara cati |
| Angiostrongyliasis | Angiostrongylus cantonensis |
| Strongyloidiasis | Strongyloides stercoralis |
| Gnathostomiasis | Gnathostoma spinigerum |
| Trichinosis | Trichinella spiralis |
| Paragonimiasis | Paragonimus westermani, Paragonimus mexicanus, Paragonimus skrjabini |
| Schistosomiasis | Schistosoma japonicum, Schistosoma mansoni, Schistosoma haematobium |
| Echinococcosis/Hydatid Disease | Echinococcus granulosus, Echinococcus multilocularis |
| Sparganosis | Spirometra sp. |
Disclaimer- The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of M3 India.
About the author of this article: Dr. Annesh Bhattacharjee is a Consultant Neurologist, Dept. of Neurology GNRC Hospital, Guwahati.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries