Onco Panel: Experts discuss role of NACT in varying resectability of oral cavity cancer
M3 India Newsdesk Jun 01, 2020
Neoadjuvant Chemotherapy (NACT) has triggered many discussions among oncologists in India. While some still question the approach involving NACT in oral cavity cancer, considering the varying nature of its resectability, some outrightly reject it to stand by old-school treatment. M3 India invited some brilliant minds from the field of oncology to hold a discussion and lay their thoughts out for our members.
One of the most common cancers in India, head and neck cancer (HNC) presents a fundamental challenge in treatment- especially, oral cavity cancer, where patients present at a locally advanced stage. Surgery can prove to be effective but may be futile due to the possibility of positive margin if performed. This particular situation is specific to India and not common in the Western World. Therefore, we try to find solutions for these patients. We use chemotherapy to reduce the size of the tumor as if the size is reduced, there is a potential that they may be resected. We have used this approach in approximately few thousand patients now.
Our observation suggests that we are able to resect approximately 40% of these patients. Patients undergoing surgery after chemotherapy have a 2 year survival of around 50% and in patients who does not respond to chemotherapy and are not able to undergo surgery the survival remains around 8 months. Our analysis also suggests that this is a safe procedure and we are able to covert a majority of the patients to margin negative when surgery is done. We strongly recommend this approach for technically resectable oral cavity cancer.
Our panelists- Dr. Kumar Prabhash, Nirmal Raut, Dr. Vanita Noronha, Dr. Vijay Patil, and Dr. Bharat Bhosale here discuss the analyses of different treatment approaches, their effectiveness after NACT, and addres other related concerns. Dr. Prabhash (Professor, HOD, Department of Medical Oncology) from TMH moderates the discussion.
Click here to read the transcript of the discussion.
Dr. Prabhash: Hello, everyone! We are about to begin an interesting discussion on neoadjuvant or induction chemotherapy in oral cavity cancer, especially in technically unresectable patients. This is a fairly common condition found in Indian patients with different clinical aspects as compared to the Western world. Today, we have a panel of esteemed clinicians, who deal with these kinds of tumours and have extensively worked in this area.
Dr. Noronha: We know from decades of practice and experience that surgery is the treatment of choice for oral cavity cancer. We don’t have level one evidence to say this but we have retrospective analyses from our trials. A trial by [1] showed that in the cohort of patients with oral cavity cancer- those who underwent surgery followed by adjuvant radiotherapy had a better chance at overall survival (OS) as opposed to patients who underwent non-surgical intervention, which is clinically relevant as well as a statistically significant. So we know that surgery is the treatment of choice in patients.
Dr. Prabhash: Though the trial compared surgical vs. non-surgical approach and it appears that overall, we are not certain which one is better or worse, in oral cavity cancer, surgery is a preferred treatment if it is possible. If you have a patient with oral cavity cancer, consider surgery and try to see if it is resectable or not resectable. Now since we know that surgery is important, we have questions about resectability.
Dr. Prabhash: How does the issue of resectability come in and how do we define it? Further, how do we define borderline resectibility or estimate what is technically unresectable or borderline resectable?
Dr. Patil: We all know that a tumour is unresectable if it involves the carotid vessel or if it is going to the prevertebral fascia, or going up to the skull base. These are absolute unresectibility criteria. But, there are other criteria which come into play because of subjectivity, which is nicely presented in an article by Keeft et al. [2]
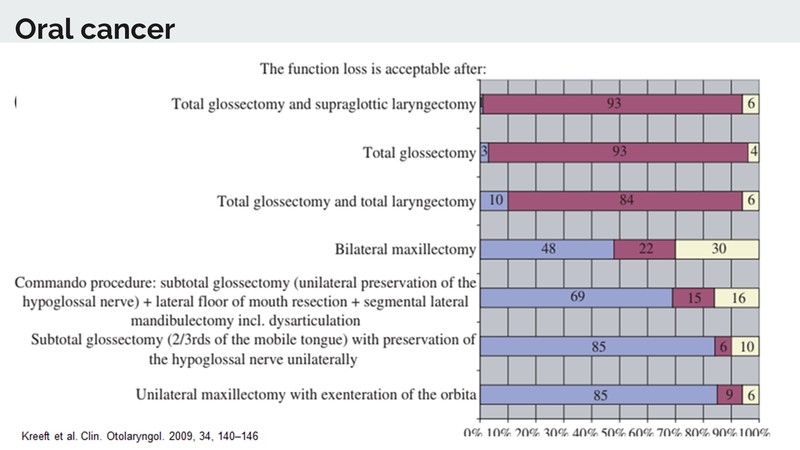
It shows that operability and non-operability may have a grey zone in between. Resectibility takes into account both, the morbidity caused by it and whether a functional reconstruction can be done. So what we see in the article [2] is that, when you ask a hundred surgeons whether they will do total glossectomy and supraglottic laryngectomy, they won’t agree. If you ask another hundred if they will do total glossectomy routinely, about 93% will say it should not be performed. If the same question is asked for total glossectomy and total laryngectomy, close to 80 to 85% will say this should not be done.
What this means is that apart from these classical criteria of unresectability, there are many other things taken into account in day-to-day practice such as: looking at the extent of resection, the morbidity, and the functional outcome with reconstruction. This is why people won’t do surgery in tumours with extensive disease.
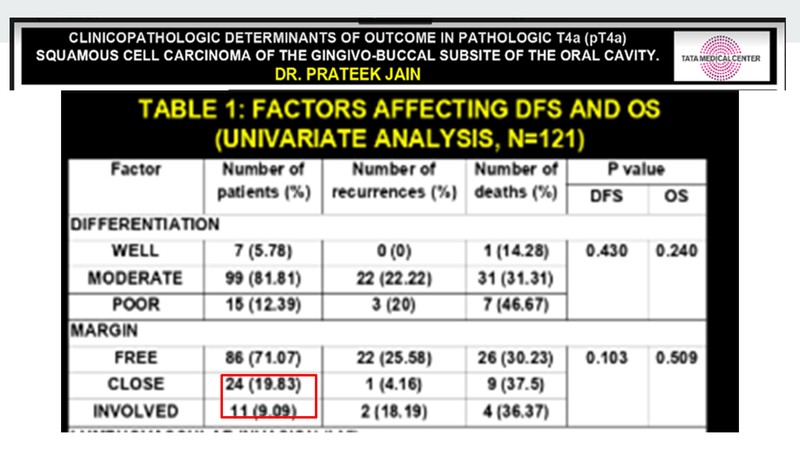
When we look at other aspects of resectibility, an analysis done by Tata Memorial Centre, Kolkata by Dr. Prateek Jain [3] on the outcome of T4a gingivo-buccal cancer comes to mind. What is interesting is that nearly 30% of the patients in the study have either closed margins or involved margins. This data is from India and is for T4a cancer.
When we look at this with respect to the data that comes from other Western countries- a slide from Dr. Jatin Shah’s lecture, which he gave at the Foundation for Head and Neck Oncology (FHNO) conference also comes to mind. It showed that in T4 tumours, in Western countries, nearly 45% were margin positive. Mind you, this is margin positive and not completion of close and margin positive as we note from the TMC Kolkata study.
When you look closely in accordance to the depth of tumour extension- if the depth is greater than 8 mm, again 45% are margin positive. It means that a large proportion of tumours apart from the classic unresectable have a high amount of margin positive rates. Margin positive rates are associated with poor outcomes. The majority of surgeons won’t operate on such tumours, especially when total glossectomy or total glossectomy laryngectomy is required or if functional reconstruction is not possible.
Dr. Sultan Pradhan, a senior surgical oncologist and the former head of Tata Memorial Centre (Surgical Department) suggested that any tumour, which has extensive skin involvement or any tumour which you would call morbid resection should be considered as borderline resectable. This has been given in an article in Adelstein et. al. [4] which revolves around the same criteria too. It states that extensive resection, in which a functional reconstruction is not possible should be considered borderline resectable.
At Tata Memorial Hospital, we have our own way of defining borderline ressectable. We give preference to achieving margin negative resection and this again is based on the extensive analysis done by Kreeft et al. [2] in which it was seen that quite a few surgeons won’t be ready to operate if they are not sure about the margins being positive or if the functional loss is unacceptable to them.
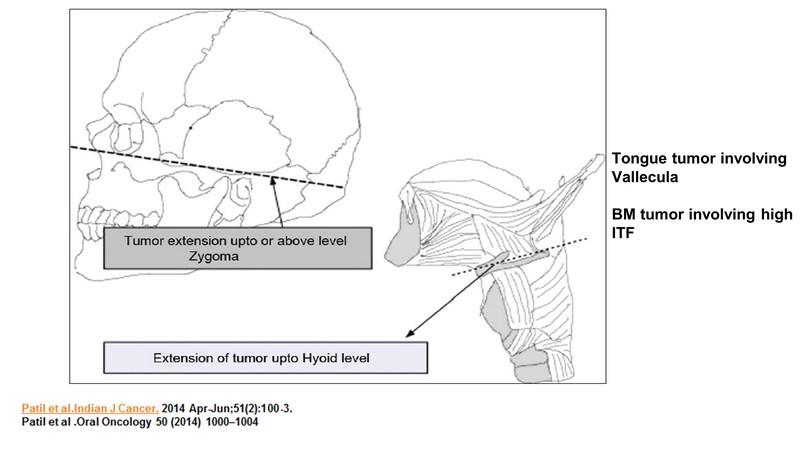
In buccal mucosa (BM) cancer, the tumour extends up to or above the level of zygoma. If the tumour involves higher invasive tumour front (ITFs), that is, in case the primary tumour is in the toungue and the disease extends up to the hyoid and is so close to it that a resection will require cutting the hyoid, which will lead to swallowing disturbances, or, if the disease has gone too close to the vallecula, in which case there would be a chance of doing a supragloticcal laryngectomy or removal of the larynx for getting a margin negative resection; it would be considered borderline resectable.
But we must understand that there should be radiological criteria and mucosal extension needs to be seen clinically or on PUA examination. So these decisions should ideally be made at a multi-disciplinary clinic. A majority of centres in India would consider neoadjuvant chemotherapy. After 2 cycles, the patient will be assessed and depending upon the response and performance status, there would be a variety of treatments decided, which could be surgery, chemoradiation, or palliative care.
Dr. Bhosale: Unfortunately, in our country we see larger tumours, so if there is an area where we have completely resectable tumours, the patients go for resection. In those who are totally palliative, we can understand that resection is not possible. But when this borderline or technically unresectable subset of a patient comes to us, there has to be collective decision made by the surgeon, radiation oncologist and other medical oncology team members. This is because, in this subset, there is an area which can become potentially resectable and as we know if a tumour in HNC is resected, the chances of cure increases.
This is the most important step in the management of HNC– to get patients out of borderline unresectable to resectability. And then comes the role of neoadjuvant chemotherapy, for which we know that there is no level one data available, but looking at the responses, we can definitely try it. As Dr. Patil mentioned, we have to sometimes figure that these patients do respond to chemotherapy and at the end of surgical resection they are into complete response (CR). As mentioned before, this is a joint decision by a multi-disciplinary oncology team.
Dr. Raut: When it comes to loco-regionally advanced oral cavity cancers, there is a difference between loco-regionally advanced, oropharyngeal and laryngeal cancers, where functional organ preservation is a very important approach. As compared to that in the oral cavity cancers, there are concerns about increased toxicity and survival advantage with the organ preservation approach. Hence, surgery forms a very important modality of treatment in oral cavity cancers. This is why we need to see which of these cancers are potentially resectable and then plan neoadjuvant chemotherapy accordingly.
Dr. Bhosale: It is also important to have a trained team- dedicated medical oncologists who treat HNC and surgeons working in high-volume head and neck cancer centres, particularly for this subset of patients. Selection plays an important role for these patients. Otherwise, most of them can become palliative.
Dr. Prabhash: Now as per the definition Dr. Vijay mentioned, does it look fair in your practice, that those patients, by and large, don't often go for surgery and instead go for an approach to downsizing tumours and then consideration for surgery in the future?
Dr. Bhosale: Yes, in my practice, we have a team, including a dedicated radiation oncologist and an HNC surgeon. Earlier, a large volume of patients would come with a thicker or bigger tumour, with extensive skin involvement. Maybe in the two-tier, three-tier cities, expertise was not available or the waiting list was full. Nowadays, fortunately, in most parts of India, we have trained HNC surgeons, so it has improved. Now, we get patients referred to us by our colleagues to consider for neoadjuvant chemotherapy.
Dr. Prabhash: HNC is one area where a multi-disciplinary approach is encouraged, unlike other cancers, where the patient gets operated and is then seen by a medical oncologist. In HNC, the patient is jointly seen or the surgeon sees the case first and refers to us for an opinion, asking if NACT would be better. Hence, we are able to deliver better results.
Dr. Bhosale: So areas where no expertise is required in terms of surgery, like high ITF especially, patients, who were initially going for non-resectability now come for neoadjuvant chemotherapy and a good fraction of patients are responding to two or three drugs. In this particular subset of ITF involvement, patients are becoming resectable. Few of them are showing pathological CR and we know that the importance of surgery and improving pathology CR in this subset are good prognostic factors. These patients do well after the surgery, obviously, after using the additional modality of radiation after chemotherapy and surgery.
Dr. Prabhash: If we do take this approach, what is the outcome for these patients with NACT?
Dr. Bhosale: We have established now that patients of HNC who undergo surgery have a better outcome, and it is definitely better as compared to non-surgical aspects be it CPRT or chemotherapy followed by CPRT. So the main aim in HNC is to bring the patient to the surgical table with a good resection margin, as getting a close or a positive margin, ultimately translates into poor OS.
We have practised neoadjuvant chemotherapy with a good response rate – to the tune of two or three drugs. The responses may vary- 40 to 60% of patients, depending upon their selection and how good their performance status is based on the presence of comorbidities. A significant chunk of these patients in the borderline resectable group come to the surgical table with the achievement of a clear surgical margin, the required margin after using neoadjuvant chemotherapy.
We have been using theis approach for the last decade now. Nowadays, it is a routine approach for this subset of patients, where oedema is extensive, the tumour is going into the vallecula, or ITF is involved. These patient cases are routinely discussed in the clinic, and after informed consent, and nutritional assessment (a very important factor) they are sent for surgery. A majority of these patients, fortunately, more than 50% come for surgical resection with a clear surgical margin. In those patients, we are able to achieve functional reconstructions too. So morbidity is also decreasing.
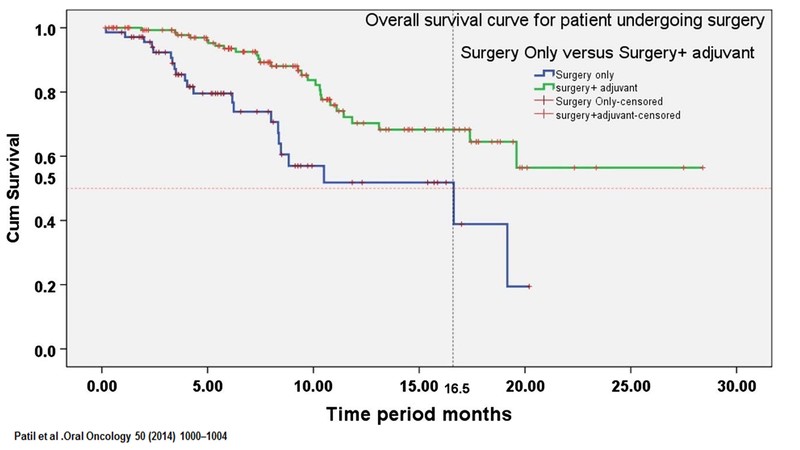
Dr. Noronha: The goal of NACT is to get the patient to surgery, which I completely agree. We had published a study Patil et al. [5] in which patients who received NACT and could then undergo surgery, had a relatively prolonged OS. The patients, who after NACT, could not undergo surgery had poor survival, which would be equivalent to the patients who received palliative therapy. So the bottom line is that the patients who undergo NACT and then undergo surgery have prolonged survival- median survival of approximately 20 months. But, patients who could not be made resectable had a median survival of about 8 months and that difference was statistically significant. So, post NACT, patients who undergo surgery have better survival. This is the significance of giving NACT to these patients.
Dr. Prabhash: Also, one of the issues remains regarding subsequent treatment. What do you do about patients who first undergo chemotherapy, respond, and then go for surgery? What do you do further for these patients?
Dr. Raut: I know some colleagues in two-tier and three-tier cities, who tell me that some senior surgeons they know review the methods of newer surgeons, who have trained from bigger institutes. Say when you send a patient to a relatively newer surgeon, he will opt for a multi-modality treatment in the form of neoadjuvant chemo, then surgery, and then post-op radiotherapy, while the older surgeon will just do the surgery and the patient will be okay with just one modality of treatment.
But, I think the message has to be very clear that just a good surgery is not enough. The role of other therapies is equally important, like the neoadjuvant therapy which will help the surgeon get a clear margin, which is a very important risk factor for recurrence. Also, treatment after the surgery (radiation or chemoradiation) is equally important. So each and every modality plays an important role in curing patients and preventing recurrence of the disease later in their lives.
Dr. Prabhash: I would like to concede to Dr. Nirmal's comment. If the tumour is upfront resectable like you have a T3N0 tumour oral cavity, it’s a good idea to resect, however, the same is not true for T3N0 larynx. The problem is that going ahead with surgery and then coming out with a positive margin is totally unacceptable. And we know that in practice, if the positive rate of margin is around 5% it is fairly acceptable. If the chance of marginal positivity is high (double digits), it is very important that we consider adding some other modalities before surgery. So I will say that it could be a disservice if in early T3N0 and T4, you decided to go ahead with surgery when you know that margin is an issue, and come out of the surgery with a margin-positive patient.
In a patient who has big tumours, if one gives NACT, followed by surgery; the patient will require adjuvant therapy. Adjuvant radiotherapy patients do far better as opposed to those who don't receive it. And the survival difference is in the tune of almost 15% as per the study by Patil et al. So it is important to remember to give radiotherapy and sunsequent chemotherapy after chemotherapy and surgery. It is important that the whole treatment is completed. It does become a bit tricky and more tedious, but it is essential.
Dr. Prabhash: We will not always have level one evidence and I always emphasise that for almost 90% of our practice. By and large, it is level three. So when you don’t have level one evidence, you work now with whatever evidence you have. Dr. Patil, can you please explain the evidence we have and put this in perspective for us?
Dr. Patil: If we look at the outcome of a surgically-resected patient, the two years outcome is around 47%. If we look at the outcome at two years for patients who underwent surgery and adjuvant chemotherapy, the outcome is around 60%. So it is safe to say that the outcome with complete treatment is 60% and the overall outcome of the cohort is in around 47%.
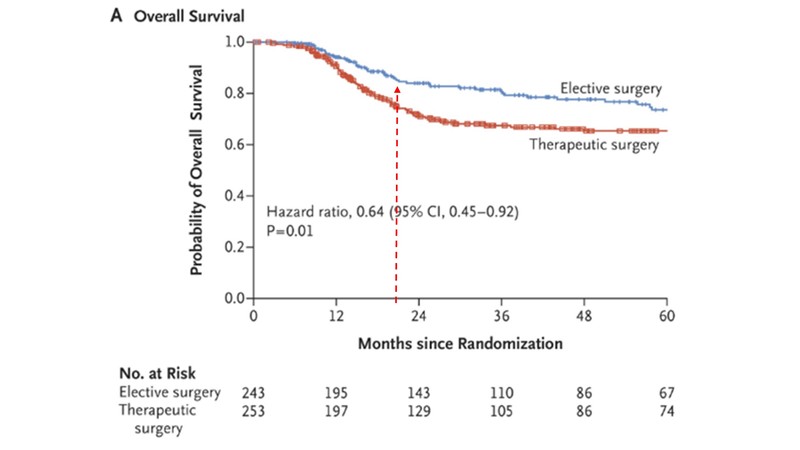
In a study by TMH, published in the New England Journal of Medicine, which was in T1,T2 clinically and radiologically node-negative mix of patients undergoing elective lymph node dissection vs. therapeutic lymph node dissection when at recurrence, what is important is the two years outcome OS which is around 80% (clinically this was stage one and stage two patients).
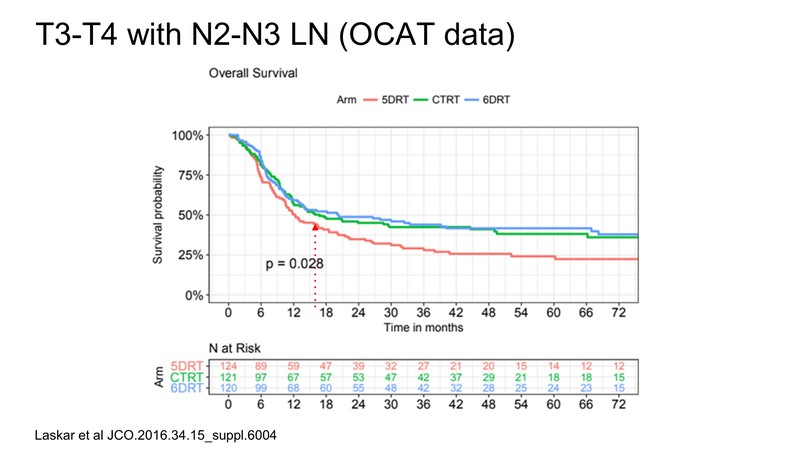
For T3,T4 patients especially, if they are node-positive, the two-year outcome comes to around 50% as per a study by Laskar et al. [6] The point is, in large T4b tumours, borderline resectable is a subset, and most of them are also node-positive. If you do NACT and after that they undergo resection, the outcomes are nearly similar to the outcomes of T3, T4 tumours, which are upfront resectable. This is a testament to the fact that by doing adjuvant chemotherapy and then surgery, we can actually downstage these tumours and then the outcome will be similar to T3 and T4 tumours.
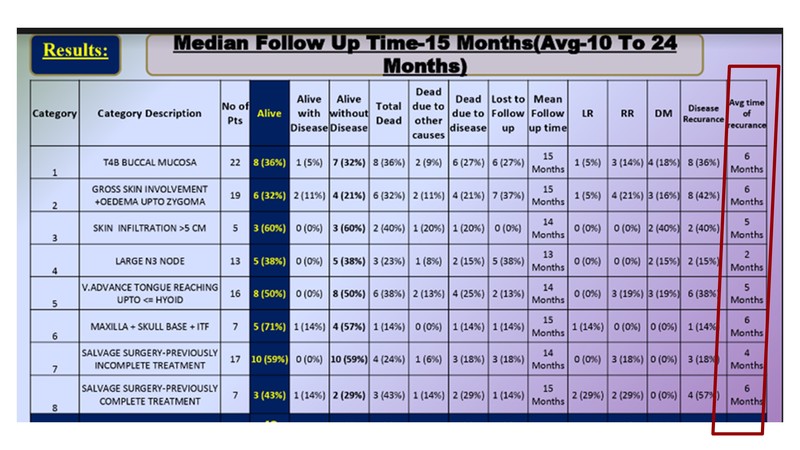
As Dr. Raut points out, a few people who rebuke the fact that borderline resectable tumours should be given NACT and instead they perform surgery. It is important to understand that you can cut anything and try to reconstruct it. Let's look at the outcomes in a few patients from a large study from Ahmedabad (unpublished but presented at FHNO and published in its supplement in 2018). Apart from criteria like gross skin involvement + oedema up to the level of zygoma, advanced tumour reaching hyoid, they have also taken all T4b tumours and skin infiltration of more than 5 cm into consideration. Results achieved by doing an upfront surgery in this large cohort of patients show that the minimum time for recurrence is 2 months and the maximum is 6 months. The study shows that these surgeries are actually futile as most of the patients failed within 2 to 6 months.
Another study published in NEJM considered the concept of doing PET CT to avoid futile surgeries. The article clearly stated that if you do surgery and the patients are experiencing recurrence within a year, it should be considered futile. If you use this definition, it means that doing surgery in borderline resectable cases is futile most of the time. The message should be clear that performing this is unlikely to yield good results and is not good for patients.
There are certain institutes, which say that if the patient is not operable, he/she can be given radical cisplatin-based chemoradiation therapy (CTRT). As pointed out, oral cancer is not a very radio-sensitive disease and obviously, large oral cancer is even less radio-sensitive. The outcomes with radical CTRT in this setting are really poor.
An analysis done from Tata hospital showed that in unresectable oral cancers, young and fit patients who had small tumours (in which you can try to be radical), had a median survival of less than 6 months (precisely 5.5 months) which again means that surgery is important. Upfront surgery, however, is a futile effort in borderline resectable cancers. So when you consider all the results discussed, those given by neoadjuvant chemotherapy are very similar to the results shown by upfront T3, T4 node-positive tumours. So this should be the approach which should be taken forward.
Dr. Bhosale: As mentioned, while we do not have level one evidence, so we must continue to practice and bring together all the evidence which is published. The numbers too are mostly Western data and as we know the tumours in patients in the West are not as big as those seen in our country. This includes skin infiltration. It is good now that in two-tier and three-tier cities, we have trained surgeons and correct decisions are being made considering the opinions of medical oncologists, radiation oncologists, as well as radiologists. This translates into a good outcome. So a patient needs to know that while surgery is the most important thing, surgery with good positive margin where no other functional reconstruction is needed means a good quality of life (QoL) because that is very important in oral cancer. You cannot have a survival of two years with bad QoL. The patient wants survival and at the same time, the patient wants good QoL. As a result, a plastic surgeon is also a very important part of this process, and it is a multi-disciplinary approach.
Dr. Patil: I agree. In HNC, the experience which we have so far is of around 721 patients. We had to slow down due to the spread of COVID-19, but will be publishing our experience based on 4,000 patients with borderline resectable outcomes soon. We must understand that in HNC, one does not get to do a randomised trial very frequently. If you look at the treatment modalities which we use, such as in early-stage cancer, we prefer surgery over radiation, so there is no randomised trial. Based on the retrospective evidence we give, radiation, adjuvant radiation in early-stage for LVI/PNI. And then there is no randomised trial. The same is the case for locally advanced HNC when we give adjuvant radiation or do surgery. Most of this data comes from a good retrospective analysis. So I completely second the fact that while we may not be getting randomised trials and data, (though that would be best), we should be accepting data, which is huge and comes from a good-quality centre.
Dr. Bhosale: We recenty published 5 papers- in patients with ITF, young patients, and patients with distant metastasis. We do see that patients who are offered the neoadjuvant chemotherapy experience less distant metastases. Those patients who undergo surgery upfront are young and are getting closer margins and more distant metastases, which is not seen in international data. So I do see an Indian subset of patients. We definitely see a potential for the role of neoadjuvant chemotherapy, and that should be practised.
Dr. Prabhash: How do we select these patients for NACT? Because we know that NACT is toxic and there's a problem. And what are the criteria we use to select them?
Dr. Raut: There are different factors which we will look into while selecting our patients for neoadjuvant chemotherapy. Some are patient-related factors, some are tumour-related factors. Another important factor is the age of the patient. Even if the patient's age is say 50 or 60, but he's nutritionally not doing so well, he will not be able to tolerate the treatment properly. Then we look at the physiological age, which is more important and the performance status of the patient. For zero to one, patients usually do well with any kind of three-drug chemotherapy. As the performance status increases, we have to choose between three drugs or two drugs. Then comorbidities like blood pressure, diabetes, drugs the patients are taking and how well controlled these comorbidities are should also be considered. The nutritional status is very important especially because the tumour is in the oral cavity and hence the food intake of this patient would be compromised and they could lose weight. Even during chemotherapy, they experience severe nausea and vomiting and to add to that if the nutrition is hampered, they don't tolerate the treatment well.
There are other factors like cardiac fitness, any pre-existing diabetic neuropathy and yes, in a country like ours, with the GDP so low, the financial aspect also plays a very important role. If you use stronger chemotherapy with three drugs, supportive care is also essential. Moreover, most patients have to travel from far to come to the hospital, so if during the treatment, the patient has neutropenia or any signs of infection, they don't report to the hospital and it proves dangerous. All these factors should be considered before we decide upon the kind of drugs we select for the patient, be it a schedule with two drugs or three drugs or using three-weekly or weekly regimens.
Dr. Prabhash: Two drugs versus three drugs- please comment.
Dr. Noronha: We have data from TAX323 and 324 (Vermorken et al.), [7] which were trials done in unresectable HNC and we know that the three-drug regimen of Docetaxel, Cisplatin and 5-FU was superior to the two-drug regimen, which had Cisplatin and 5-FU. We do not have level-one evidence, in which there is a platinum and a taxane of two-drug regimen compared to a three-drug regimen, yet. But we had retrospectively analysed our two-drug regimen, which was a non-5-FU two-drug regimen.
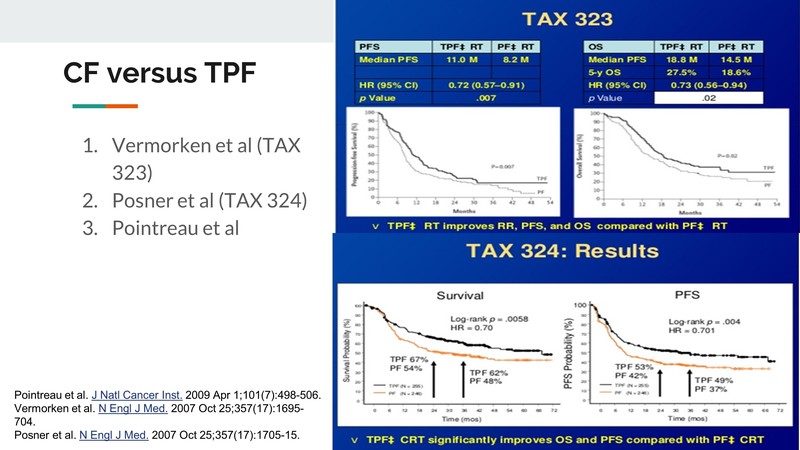
So when comparing taxane and platinum versus the three-drug regimen of taxane, platinum and 5-FU, we found that the three-drug regimen led to a superior response rate and superior survival. We also found that when choosing the two-drug regimen, Docetaxel was better than Paclitaxel; the type of platinum did not matter. Carboplatin or Cisplatin- both led to a similar outcome, but Docetaxel was important. And if you can choose a three-drug regimen, a three-drug regimen would be better than choosing a two-drug regimen.
Dr. Prabhash: Can you please comment further on a pharmacogenomics in our practice when you give NACT, and does it help?
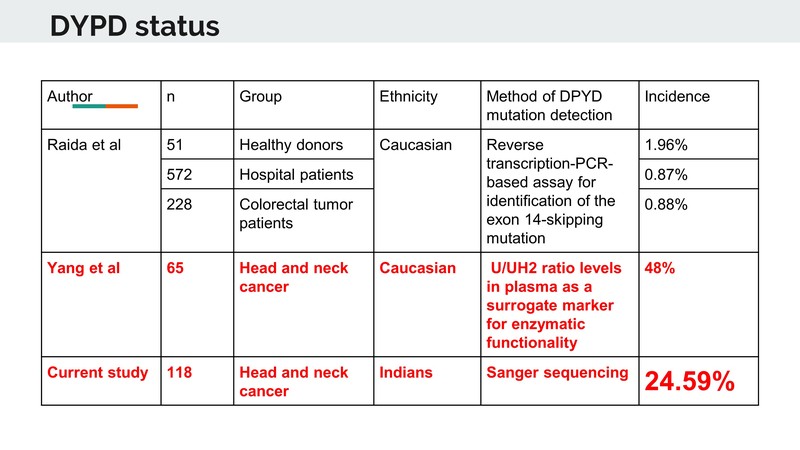
Dr. Noronha: In pharmacogenomics, specifically talking about the Dihydropyrimidine Dehydrogenase Enzyme, in international literature, the prevalence and incidence of DPD deficiency if heterozygous and homozygous is very low. We found a significant level of toxicity in our patients who were receiving 5-FU and so we took our patients prospectively who developed severe toxicities, who had received a 5-FU regimen in the NACT setting and we found that a majority of the patients who developed severe toxicities had some form of DPD deficiency. The majority were heterozygous, but there were a few who were homozygous as well. We took this forward and in a hundred-odd patient cohort, we evaluated the incidents of DPD deficiency. We found that approximately 25% of our patients had a DPD enzyme deficiency. So while in the international literature it is in single digits, say 1 or 2%, we found a 25% deficiency in our patients. And that maybe because 5-FU containing regimens lead to excessive toxicity.
Dr. Prabhash: Would anyone else like to comment?
Dr. Bhosale: We have not been doing it as a routine, but I had come across a few patients who had developed toxicity to 5-FU especially, but that was not a large number. But yes, when such data comes from a big institute, we cannot neglect it. Recently, I have started prescribing 5-FU because sometimes if you have a homozygous mutation patient, there is a possibility of severe toxicity and with some cases, it leads to mortality too. So these things need be considered while selecting patients for toxic therapies.
Dr. Raut: I think now when you are using a three-drug regimen, it becomes toxic, and using these pharmacogenomics is very important. But the problem in private clinics, as Dr. Bhosale mentioned is that we have to really think about how many tests we are going to order, the cost and the wait time for getting the report. These are some of the reasons why we don’t do it on a routine basis. But yes, looking at the data, we can see it is as high as 25%, so 1 in 4 patients is going to have this particular mutation. This is why we need to be really vigilant about using 5-FU.
Dr. Prabhash: The next question in this approach of treating Oral Cavity Cancer is how do we evaluate those NACT responses? Is there a trick to it and if you should do it.
Dr. Patil: Well, it has to be both clinical and radiological. That's what a study from Tata Hospital stated and it said that we select only those patients, who had a near-pathological CR or CR. The data showed that quite a few of these patients were reported not having stable disease and surprisingly, one or two even having progressive diseases. What it means is that when we looked at the correlation between the radiological response and the pathological response, we didn’t find a very good correlation. But when you ask the patient if he or she felt if the disease had decreased after the start of chemotherapy, surprisingly, there was a linear correlation between what the patients told us- that it had decreased after chemotherapy.
This was an example. So when you look at the scan of this particular patient, the patient has a borderline resectable disease, a borderline resectable disease, a large fungating mass and oedema as well. When they look at it after two cycles of NACT, the disease has shrunk considerably, oedema has also come down, the mucosal extent of the disease has nearly disappeared and there is only a bit of a scar left.
Clinically, I would say that it's a very good partial response. Some of us may even say that it is a near-CR. Now the problem is the scan, even of this patient, is not showing near-CR. The reason is the way we assess radiology with RESIST criteria and with that, we don't look at the biology of the disease, but look at the morphology and sizes. For sizes, we look at the longest dimension in the plane in which the imaging has been done and in most situations, it is done in axial imaging. So we look at the longest dimension, which is in the axial image.
So if the tumour is like situation A, which is a nice spherical tumour and if it shrinks concentrically, you would definitely see a decrement reported even in RESIST.
If we take situation B, which has an elliptical or a cylindrical tumour, even if there is good shrinkage, we might not find a good volumetric shrinkage. You won’t find good shrinkage when you label it according to RESIST.
If you look at situation C, in which there is an irregular tumour size and the tumour has to go between facial planes and bones and it's toward different places (which is the most common situation in borderline resectable HNC as shown in the radiological markings of the tumour), you take the longest diameter and even if there's a shrinkage in one axis, and if this doesn't reflect in all dimensions, RESIST fails to document it.
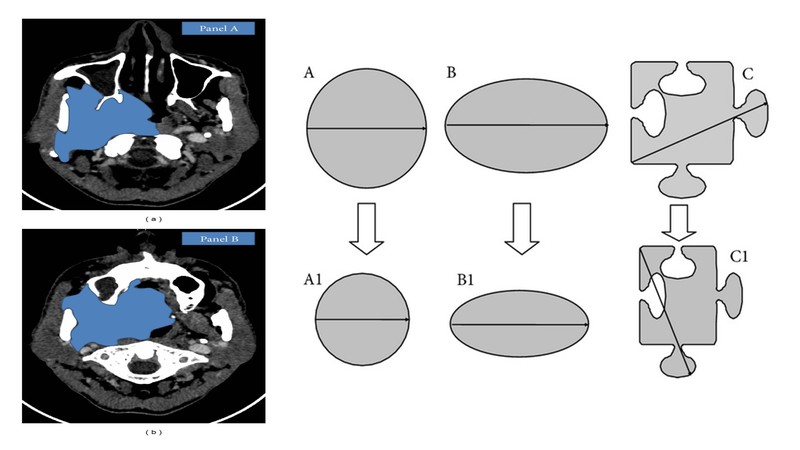
Hence, it is very important to understand that when you're doing response assessment of these patients you need to have photographs of previous prior treatment and post-treatment, and you need to do a good clinical examination. You need to have a subjective history from the patient, how the patient is doing, definitely radiology, etc. It is clinico-radiological responses which works best in borderline resectable HNC and not only radiological responses as Dr. Bhosale has also pointed out. Many of the stable diseases actually turned out to be pathological responses. So it has to be clinical-radiological way of doing response assessment.
Dr. Bhosale: The message for our viewers is: You have to keep the basic principle of medicine, history-taking and clinical examination. What I do know, unfortunately in some cases, one starts the chemotherapy and then assesses the patient after two cycles. That should not happen. Periodic assessment of the patient is very important. One needs to assess the patient, know the status clinically, and make a judgment. As we have seen in some of the cases, radiological responses were not documented, but at the end of surgery the patient has a pathological CR. So that subset of patients should not be missing an opportunity of cure. So assessment is very important clinically as well as for history-taking.
Dr. Prabhash: So these were good comments by Dr. Bhosale and Dr. Patil. Remember why you are giving chemotherapy. We don't have better criteria than RESIST so we need to live with this. But here the aim changes. If your tumour is up to zygoma and you are giving chemotherapy, the moment you have a reduction, which gives you a centimetre to cut it, you will have to go ahead with it. If you have a tumour you're giving for NACT because it is close to hyoid, the moment you have a margin of a centimetre or less than 0.7 or 0.8, you could go ahead with surgery. That time you don't go by RESIST having responded or not. And Dr. Patil highlighted the fallacy of RESIST in HNC. I concede. We have a better way to evaluate the response and we continue to use RESIST. We need to keep in mind here- the moment the criteria gets removed by chemotherapy, we know that a patient can go for surgery. So then we don't wait for RESIST-based criteria for response evaluation to decide for surgery. That should be kept in mind.
Dr. Prabhash: Now what happens after NACT is given. I'll request Dr. Noronha to comment on it.
Dr. Noronha: This was our retrospective evaluation of 700 odd patients (Patil et al.) [5] who were treated with borderline resectable oral cavity cancers and who received neoadjuvant chemotherapy. Three hundred patients were able to undergo surgery, while 400 patients underwent non-surgical therapy. Following surgery, there were about 200 patients, who could receive adjuvant chemoradiotherapy, while a 100-odd patients could not receive adjuvant chemo radiotherapy. In the non-surgical group, a small number of patients were treated with radical intent chemo radiotherapy, while the majority of patients (2/3rd) went forward for palliative intent therapy.
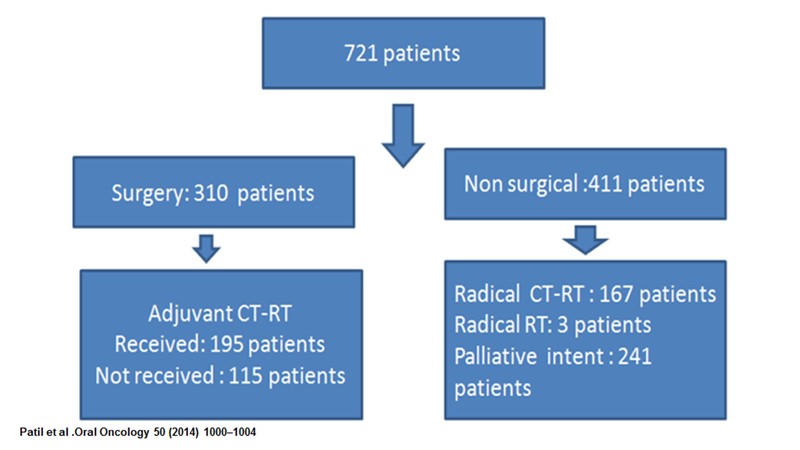
We found that in patients who could undergo surgery following neoadjuvant chemotherapy, the vast majority (97%) of patients had a margin negative R0 resection. Close margins were present in 3% of patients. There were no involved margins. About 50% of half of them were lymph node-positive and in terms of other poor prognostic features, a third of the patients had perinodal extension and less than 10% of patients had perineural invasion or lymph vascular emboli.
To re-emphasise, what happens to the margins after neoadjuvant chemotherapy; the vast majority of patients could have margin negative resection. In patients who underwent upfront surgery, the percentage of patients who had positive margins as compared to the patients who underwent surgery in the borderline resectable group who receive neoadjuvant chemotherapy followed by surgery were of a similar proportion for negative resection. That's the bottomline- all borderline resectable patients who underwent neoadjuvant therapy and could then undergo resection had R0 margin negative resections.
Dr. Prabhash: The second part of what Dr. Vanita also commented on the complications. What happens after giving neoadjuvant chemotherapy, and you do surgery? A study conducted by Joshi et al. [7] compared T4 tumour with upfront surgery versus T4 tumour with chemo chemotherapy followed by surgery and it looks like surgical complication in both the groups is similar. So, neoadjuvant chemotherapy doesn't increase complication in borderline resectable patients when we are giving this neoadjuvant chemotherapy to make them resectable.
Dr. Prabhash K.: Any comment regarding post-op complications or margins in their practice?
Dr. Bhosale: So I look at an opportunity of giving neoadjuvant chemotherapy. You buy time for patients who are nutritionally deprived; you can add that nutritional intervention while giving neoadjuvant chemotherapy. I see the improvement in their performance status. If you look at the geriatric population or those with a larger tumour, of course, after starting neoadjuvant chemotherapy, there will be a dramatic improvement in the performance status as well as the nutritional status. You buy that time to improve the status, which adds to tolerance to the surgical intervention as well as whatever the patient is going to face after surgery- adjuvant radiation.
So in my subset of patients, they improve clinically and I see lesser complications. The competition rate is increased by adding neoadjuvant chemotherapy and at the same time, you are able to achieve a higher number of clear margins. So there is definitely a peer role of neoadjuvant chemo in this particular subset of patients.
Dr. Raut: Talking about postoperative therapy after surgery, I think that also is an important part of the treatment. After the surgery we need to give these patients adjuvant chemo radiation along with chemotherapy. Here it becomes a little challenging because these patients have received neoadjuvant chemotherapy, which is platinum-based and also after that undergone surgery with a lot of drugs, antibiotics, etc. That is where we need to be really careful, while selecting our drugs along with radiation.
Along with radiation, we usually give Cisplatin, which could be weekly or three-weekly, but we need to check the age of the patient, the GFR, the kidney status, and then select the drug accordingly. We could, maybe, choose a biological therapy like Cetuximab or may be at times omit the drug because the chances of having kidney toxicity could be higher. So that is why it is sometimes as simple as just chemo radiotherapy, because the patient has been treated earlier and has received platinum-based chemotherapy.
Dr. Bhosale: It is very important what you give in the neoadjuvant setting but pertaining to the surgery you get to buy time to build up the nutrition status of the patient. As Dr. Raut rightly pointed out, if you use cisplatin and have given it for more than two or three cycles, to use this again is a big challenge. You have to do repeated audiometries and will have to monitor the creatinine clearance. Perhaps, Dr. Prabhash or Dr. Noronha can guide us on that.
Dr. Prabhash: What are the options we have? We give weekly Cisplatin, so that is one option, but there is a chance that there may be more toxicity. A study compared Cisplatin vs Cetuximab which is a targeted therapy and what was was realised was that if you compare them, the kidney dysfunction with Cisplatin goes down significantly if you use Cetuximab. If you can’t use Cetuximab, don’t forget, you do have carboplatin single agent, which can be thought about, but you do have cetuximab in this setting.
Dr. Prabhash: In conclusion, can we have remarks from each panellist regarding the tumour, stage and treatment approach which we are taking?
Dr. Raut: Oral cavity cancers are very important from the Indian perspective and surgery forms the mainstay of treatment. Multi-modality treatment is very important to ensure that there is no recurrence in the management of oral cavity cancers.
Dr. Bhosale: In HNC, bringing a patient to the surgical table is very important, but surgery is not the only thing which is curative. We have to add neoadjuvant chemotherapy in the adjuvant setting, chemotherapy and radiation therapy to achieve the longest possible survival as well as reduction of the loco-regional recurrence. So a multi-modality treatment decision needs to be taken at the beginning, when we see the patients. And there has to be interaction among the surgeon, plastic surgeon, medical oncologist, radiation oncologist, and radiologist. That I think, will definitely help in improving the patient’s survival.
Dr. Noronha: Patient selection is of paramount importance and for patients with technically unresectable oral cavity cancers, neoadjuvant chemotherapy improves the resectability and outcome. Hence, it must be considered.
Dr. Patil: I agree with everyone.
Dr. Prabhash: In conclusion, what we have emphasised here is that if you have technically unresectable patients, it is good to have some criteria in your clinic. If they are borderline or technically unresectible, consider giving them some therapy initially, including neoadjuvant chemotherapy. The patients who respond can be taken for surgery; that can be almost half of them. Then followed by some adjuvant therapy, preferably chemo radiation, give Cisplatin or Carboplatin, or other available therapies. This will improve outcome of these patients as compared to a group treated with palliative systemic therapy, palliative radiotherapy or chemoradiation.
Thank you, everyone, for your inputs and I hope the audience will find it valuable. Thank you!
References:
[1] Iyer NG et al. Randomized trial comparing surgery and adjuvant radiotherapy versus concurrent chemoradiotherapy in patients with advanced, nonmetastatic squamous cell carcinoma of the head and neck: 10‐year update and subset analysis. Cancer. 2015 May 15;121(10):1599-607
[2] Kreeft et al. The surgical dilemma of ‘functional inoperability’ in oral and oropharyngeal cancer: current consensus on operability with regard to functional results. Clin. Otolaryngology. 2009. 34, 140-146
[3] Jain PV, Sharan R, Manikantan K, et al. Clinicopathologic Determinants of Outcome in Pathologic T4a (pT4a) Squamous Cell Carcinoma of the Gingivobuccal Subsite of the Oral Cavity. Indian J Surg Oncol. 2019;10(4):594‐599
[4] Adelstein et al. An Intergroup Phase III Comparison of Standard Radiation Therapy and Two Schedules of Concurrent Chemoradiotherapy in Patients With Unresectable Squamous Cell Head and Neck Cance. Journal of Clinical Oncology. 21, no. 1 (January 01, 2003) 92-98.
[5] Patil VM, Prabhash K, Noronha V, et al. Neoadjuvant chemotherapy followed by surgery in very locally advanced technically unresectable oral cavity cancers. Oral Oncol. 2014;50(10):1000‐1004
[6] Laskar et al. Phase III randomized trial of surgery followed by conventional radiotherapy (5 fr/Wk) (Arm A) vs concurrent chemoradiotherapy (Arm B) vs accelerated radiotherapy (6fr/Wk) (Arm C) in locally advanced, stage III and IV, resectable, squamous cell carcinoma of oral cavity- oral cavity adjuvant therapy (OCAT): Final results (NCT00193843). Journal of Clinical Oncology. 34, no. 15_suppl (May 20, 2016) 6004-6004
[7] Joshi et al. Comparison of postoperative complications in advanced head and neck cancer patients receiving neoadjuvant chemotherapy followed by surgery versus surgery alone. Indian J Med Paeditr Oncol. 2015. 36(4): 249-54
Disclaimers:
This document is a transcription of the video, produced for audience with bandwidth limitations that could possibly restrict them from viewing it. While it is believed to be accurate, it is not warranted to be so. Divergence in format is to be expected.
The views and opinions expressed in this article are those of the speakers and do not necessarily reflect the official policy or position of M3 India.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries