Latest approach to bronchial asthma management: Dr. Jyotsna Joshi
M3 India Newsdesk Mar 25, 2019
Dr. Jyotsna Joshi explains the classification and goals and steps in management of bronchial asthma based on the GINA guidelines; variable dose therapy, immunotherapy, and monitoring of treatment in patients.

Bronchial asthma is a chronic inflammatory disorder of the airways associated with airway hyperresponsiveness that leads to recurrent episodes of wheezing, breathlessness, chest tightness and coughing particularly at night/in the early morning. These episodes are usually associated with widespread, but variable airflow obstruction that is often reversible either spontaneously or with treatment. This review deals with the latest approach to asthma management.
Diagnosis of asthma
Diagnosis of asthma is considered in a patient who complains of dyspnoea with wheeze, chest tightness, and cough. These symptoms tend to be variable, initially intermittent and later persistent, particularly if untreated.
- History of other atopic conditions i.e. allergic rhinitis, asthma, atopic dermatitis, eczema and urticaria in the family supports the diagnosis of asthma.
- Additional history of symptoms of gastro-oesophageal reflux disorder exercise-induced worsening, nocturnal worsening, aspirin (or another drug) hypersensitivity helps in assessing severity and making treatment decisions.
- History of medications for other medical conditions particularly systemic hypertension (B blockers or ACE inhibitors) must be elicited. Physical examination is usually unremarkable except the presence of rhonchi on auscultation.
- Laboratory tests are unremarkable except eosinophilia and elevated serum IgE levels.
- Lung function testing is important.
- Peak expiratory flow (PEF) can be performed by most adults and children, even as young as 5 years of age. PEF can be used for diagnosis if the PEF increases more than 15 per cent 15 to 20 minutes after inhalation of rapid-acting β2-agonist. It is important to establish a personal-best value.
- Spirometry is useful in corroborating a diagnosis of asthma and in providing a longitudinal follow up of a given case. Spirometry in an asthmatic patient typically shows an obstructive abnormality that improves with bronchodilator therapy i.e. reduced FEV1/FVC ratio, reduced FEV1 and improvement in FEV1 after administration of inhaled bronchodilator by 12% and 200 ml.
Classification of asthma by severity
Global Initiative for Asthma (GINA) guidelines has classified bronchial asthma as intermittent mild persistent, moderate persistent or severe persistent depending on the severity of symptoms, peak expiratory flow rate and FEV1.
Classification of asthma (modified from GINA guidelines)
- Intermittent asthma is characterized as follows:
- Symptoms of cough, wheezing, chest tightness, or difficulty breathing less than twice a week
- Flare-ups are brief, but intensity may vary
- Nighttime symptoms less than twice a month
- No symptoms between flare-ups
- Lung function test FEV 1 is 80% or more above normal values
- Peak flow has less than 20% variability am-to-am or am-to-pm, day-to-day
- Mild persistent asthma is characterized as follows:
- Symptoms of cough, wheezing, chest tightness, or difficulty breathing 3-6 times a week
- Flare-ups may affect activity level
- Nighttime symptoms 3-4 times a month
- Lung function test FEV 1 is 80% or more above normal values
- Peak flow has less than 20-30% variability
- Moderate persistent asthma is characterized as follows:
- Symptoms of cough, wheezing, chest tightness, or difficulty breathing daily
- Flare-ups may affect activity level
- Nighttime symptoms 5 or more times a month
- Lung function test FEV 1 is above 60% but below 80% of normal values
- Peak flow has more than 30% variability
- Severe persistent asthma is characterized as follows:
- Symptoms of cough, wheezing, chest tightness, or difficulty breathing that are continual
- Frequent nighttime symptoms
- Lung function test FEV 1 is 60% or less of normal values
- Peak flow has more than 30% variability
Management of Asthma
The goals of asthma are:
- Minimal or no symptoms including nighttime symptoms
- Minimal asthma episodes or attacks
- No emergency visits to physicians or hospital
- Minimal need for reliever medications
- No limitations on physical activities and exercise
- Near normal lung formation
- Minimal or no side effects from medication
Prevention: Sensitisation to inhalant allergens, including dust mite/mould spores/cat/dog/other animal proteins cockroach and other insects, as well as outdoor pollens, is common among asthmatic patients. Routine measures of dust control and avoidance of obvious triggers are important. An elaborate search for the offending allergens and avoidance is cumbersome and not necessary. Simple measures to avoid exposure to these allergens are
Common asthma risk factors and action to reduce exposure
- Domestic dust mite allergen:
- Wash bed linens and blankets weekly in hot water and dry in a hot dryer or the sun
- Encase pillows and mattresses in airtight covers
- Replace carpets with linoleum wood flooring especially sleeping room
- Use leather or plain wooden furniture instead of fabric upholstered furniture. If possible, use a vacuum cleaner with filters
- Allergen from animals with fur:
- Remove animals from the home, or at least from the sleeping area
- Cockroach/insect allergy:
- Clean the home thoroughly and often
- Use pesticide spray but make sure the patient is not at home when spraying
- Outdoor pollen, mould and pollution
- Air conditioning can reduce outdoor pollen and pollution
- Reduce dampness in the home; clean any damp areas frequently
However, patients with asthma at any level of severity should avoid:
- Exposure to environmental tobacco smoke
- Exposure to air pollution
- Use of beta-blockers. ACE inhibitors and nonsteroidal anti-inflammatory drugs ( NSAIDs)
- Sulfite-containing containing food and beverages
Pneumococcal polysaccharide vaccine (PPSV 23) given once and repeated after 65 years of age and yearly influenza vaccines are also recommended.
Immunotherapy
Immunotherapy can be given in the form of hyposensitisation to allergens either by subcutaneous immunotherapy (SCIT) or sublingual immunotherapy (SLIT). SLIT has been recommended for adult patients with rhinitis and allergic to house dust mite with exacerbations despite ICS provided FEV1 is >70% predicted.
Biologicals like anti-IgE (omalizumab) for severe allergic asthma ≥6 years and anti-IL5 (SC mepolizumab or IV reslizumab) for severe eosinophilic asthma (age ≥12 years) may be used as add on therapy in step 5.
Pharmacotherapy
- Relievers: short-acting beta 2 agonists like salbutamol as required
- Preventers: anti-inflammatory like inhaled corticosteroids
- Controller: add on drugs like tiotropium, theophyllines and anti-leukotrienes like montelukast
The overall strategy is to use a stepwise approach based on the level of severity. “Reliever” or “rescue” medications in the form of inhaled short-acting β2-agonist (SABA) are used on as needed basis is recommended for patients with very mild intermittent asthma who are asymptomatic between episodes.
Patients with persistent asthma (mild, moderate or severe) are in addition treated with anti-inflammatory agents or “preventer” medication primarily inhaled corticosteroids.
Patients with persistent asthma, with more frequent symptoms, are treated with the addition of anti-inflammatory agents, inhaled corticosteroids (ICS), “preventer” medication is used on a scheduled basis, along with reliever or rescue medications, on an as-needed basis.
Regular use of ICS apart from anti-inflammatory action can also prevent asthma exacerbation, increase in bronchial hyperresponsiveness and accelerated loss of lung function. It also reduces mortality due to asthma.
Additional, “controller medications” like long-acting β2-agonists (LABA, theophyllines, anticholinergics (ipratropium, tiotropium) and anti-leukotrienes like montelukast if asthma remains uncontrolled with ICS and SABA. These agents are also a rational alternative, taken in combination with inhaled steroids, in patients who remain symptomatic on low - intermediate inhaled steroid treatment.
The benefits of combination therapy, as measured by symptom scores, as required use of beta-agonists, lung function, and exacerbation rates, with these other agents are not as dramatic, however, as with the addition of the long-acting inhaled bronchodilator.
A step-care approach (GINA) aims to abolish symptoms as soon as possible and to optimize peak flow by starting treatment at the level most likely to achieve this.
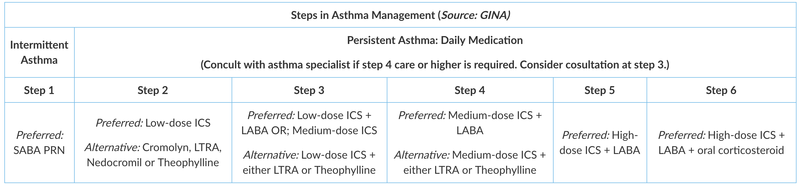
Patients should start treatment at the step most appropriate to the initial severity of their asthma. The approach allows early control, which is then maintained by stepping up treatment as necessary and stepping down when control is good (if control is sustained for at least 3 months). The goal is to decrease treatment to the beast medication necessary to maintain control. Review treatment every 3-6 months once asthma is under control.
Variable dose therapy
Variable dose therapy is an easy way of managing asthma within the framework of step care management. It involves the use of a single inhaler of fixed dose combination of low dose ICS and formoterol as the preferred LABA due to its rapid onset of action. Variable dose therapy consists of:
- SMART (Single Maintenance and Reliever therapy)
- Adjustable dose therapy where the inhaler is used to step up and step down therapy by increasing or decreasing the number of inhalations
For example, MDI Budesonide 200 mcg and Formoterol 6 mcg is given as 2 inhalations twice daily (maintenance dose) and 1-2 inhalations as required (reliever doses). Thus single inhaler is used for maintenance and reliever therapy (SMART).
Similarly, the same inhaler may be adjusted upwards to 4 inhalations twice daily if asthma is uncontrolled and stepped down to 2 inhalations twice daily once asthma is controlled (adjustable dose therapy).
The SMART therapy apart from reliever doses of bronchodilator formoterol; helps by early anti-inflammatory intervention in the form of additional doses of ICS and maintains good control thus preventing exacerbations.
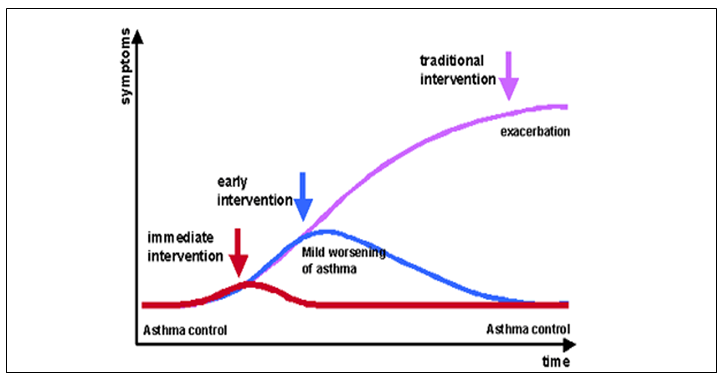
Adjustable dosing regulates convenient asthma control with a single inhaler and allowing the ICS doses to be as recommended for the appropriate step/ severity of asthma.
The doses of various inhaled corticosteroids are as shown in the table below.
| Equipotent Doses of ICS (Source: GINA) | |||
| Drug | Low Dose (µg)↑ | Medium Daily Dose (µg)↑ | High Daily Dose (µg)↑ |
| Beclomethasone dipropionate | 200 - 500 | >500 - 1000 | >1000 - 2000 |
| Budesonide* | 200 - 400 | >400 - 800 | >800 - 1600 |
| Ciclesonide* | 80 - 160 | >160 - 320 | >320 - 1200 |
| Flunisolide | 500 - 1000 | >1000 - 2000 | >2000 |
| Fluticasone propionate | 100 - 250 | >250 - 500 | >500 - 1000 |
| Mometasone furoate* | 200 - 400 | >400 - 800 | >800 - 1200 |
| Triamcinolone acetonide | 400 - 1000 | >1000 - 2000 | >2000 |
- In children above 6 years, the same dosing as adults may be considered.
- In children below 5 years of age ICS and SABA, half the adult recommended doses of ICS and salbutamol 200 mcg 4 times a day and as required is recommended.
- Add on therapy in the form of tiotropium 18 mcg at night and oral low dose sustained-release theophylline eg. Deriphyllin Retard 150 mg twice daily may be given if adequate control is not achieved.
- Short bursts of oral corticosteroids may be given if there is an acute flare-up of asthma as indicated by worsening of symptoms despite optimum complaint treatment, fall in PEF by greater than 30% and nocturnal symptoms. This is best given as prednisolone 0.6 mg/kg per day in a single dose post breakfast for 5- 7 days. Divided doses or tapering is not required. Other formulations of steroids are not superior to prednisolone.
Step down of therapy
Stepping down of therapy is equally important. Once optimum therapy is initiated, step down may be considered after 3 months except for oral steroids. Oral theophylline is stopped first, followed by tiotropium and ICS+ LABA last. Once a moderate dose of ICS is reached, then combination therapy may be stopped and low dose ICS with reliever salbutamol as required is the lowest step that is desirable.
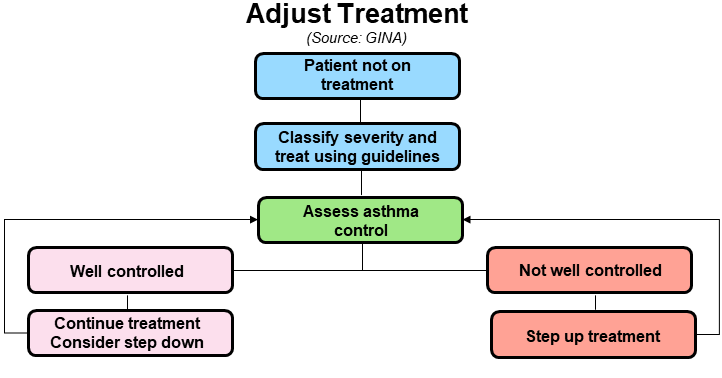
Monitoring of Asthma
Monitoring of asthma control is best done by symptoms, Asthma Control Test (ACT) and PEF monitoring. Adjustment of therapy is a continuous process and involves gauging asthma control and appropriate step up or step down of therapy.
Adjustment of therapy is a continuous process and involves gauging asthma control and appropriate step up or step down of therapy.
Asthma COPD overlap (ACO)
Asthma and COPD are the pulmonary diseases most frequently encountered in clinical practice. Usually, each disease is caused by a different aetiology and shows a different clinical picture and course. However, these two diseases sometimes present within the same patient, and it is now recognized that asthma and COPD can coexist as asthma–COPD overlap (ACO).
ACO could be diagnosed when the three major criteria and at least one minor criterion were satisfied.
Major
- Persistent airflow limitation [post-bronchodilator FEV1/FVC <0.70] in individuals 40 years of age or older
- At least 10 pack-years of tobacco smoking OR equivalent indoor or outdoor air pollution exposure [e.g. biomass]
- Documented history of asthma before 40 years of age OR BDR of >400 mL in FEV1
Minor
- Documented history of atopy or allergic rhinitis
- BDR of FEV1 ≥200 mL and 12% from baseline values on 2 or more visits
- Peripheral blood eosinophil count of ≥300 cells μL
The presence of all three major criteria and at least one minor criterion are required for the diagnosis of ACO.
Smoking cessation and appropriate vaccinations are cornerstone therapies, and pharmacologic therapy should focus on bronchodilators and inhaled corticosteroids.
Asthma comorbidities
The comorbidities of asthma have become recognized more and more in the past several years. Nearly half of adult patients with asthma had at least one medical comorbidity that was associated with poor asthma outcomes. For example,
- Patients with asthma and depression have an 11-fold increase in the likelihood of an asthma exacerbation.
- Patients who have gastrointestinal reflux disease (GERD) have a fivefold increase in exacerbation.
- Patients with obstructive sleep apnea (OSA) have a fourfold increase in exacerbation.
There is probably an even stronger association between asthma and rhinitis. About 80 to 90% of patients with asthma have some form of rhinitis or rhinosinusitis, and approximately 40% of patients with chronic rhinitis have asthma. Identifying these comorbidities and treating them is crucial in asthma management. Unlike previously believed, treating these comorbidities though important does not improve asthma control.
Asthma action plan
All people with asthma should have an asthma action plan. An asthma action plan (also called a management plan) is a written plan that is developed with the treating physician to help control asthma optimally.
The asthma action plan shows the daily treatment, such as what kind of medicines to take and when to take them. Your plan describes how to control asthma long term and how to handle worsening asthma, or attacks. The plan explains when to call the doctor or go to the emergency room.
If a child has asthma, all of the people who care for him or her should know about the child’s asthma action plan. These caregivers include babysitters and workers at daycare centres, schools, and camps. These caretakers can help your child follow his or her action plan.
Disclaimer- The views and opinions expressed in this article are those of the author's and do not necessarily reflect the official policy or position of M3 India.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries