Know Common Medicines Prohibited by the Union Health Ministry Recently
M3 India Newsdesk Jul 22, 2023
The article reports that the Indian government has banned 14 Fixed Dose Combination (FDC) medications, including those for cough, cold, and fever, based on an expert committee's finding of no therapeutic justification and potential risks. The article also highlights the banned medications.
Based on the advice of an expert group, the Health Ministry reissued the ban after it was contested in court by pharmaceutical corporations.
With immediate effect, the Indian government has outlawed 14 Fixed Dose Combination (FDC) medications, some of which include paracetamol and codeine syrup.
The Ministry of Health and Family Welfare issued a notice to this effect on June 2. Drugs known as Fixed Dose Combinations, or FDCs, are combinations of one or more pharmaceutical compounds intended to treat a specific symptom or indication.
The majority of the medications on the Health Ministry's list are ones that treat the symptoms of a cough, cold, and fever.
What is an FDC drug?
Medicines have a crucial role in healthcare. Drug combinations are routinely used to treat either a single illness or a number of comorbid diseases. Fixed dose combos (FDCs), which are often used to mix two or more medications in a single dosage form, are known.
The FDCs are justified when they show clear advantages in terms of:
- Enhancing therapeutic efficacy
- Lowering the incidence of adverse drug reactions
- Having an advantage in terms of pharmacokinetics
- Improving compliance by reducing the number of pills required
- Reducing the dose of individual drugs
- Reducing the development of resistance
- Being more affordable than individual drugs due to lower costs from packaging to distribution
It is crucial that the aforementioned assertions be sufficiently backed by scientific data.
Issues with fixed-dose combinations
Without proper care, the formulation of FDCs can result in a number of issues, including:
- A pharmacodynamic mismatch between the two components, where one drug has an additive or antagonistic effect that reduces efficacy or increases toxicity
- Pharmacokinetic mismatch and peak efficacy at different times
- Chemical incompatibility that reduces shelf life
- Drug interactions due to shared metabolising pathways
- Limitations of finer dosing titration of individual drug
Despite the fact that FDCs are accessible in practically all therapeutic areas, many of them involve strange pairings.
Cough, cold, and fever medications, analgesics and muscle relaxants, antimicrobials, medications for hypertension, dyslipidemia, diabetes, and mental disorders, as well as vitamins and minerals, are among the therapeutic groups with a large number of FDCs.
The FDC formulation may have up to five substances, or possibly more, with or without justification for their inclusion.
Government rationale for banning 14 medications
When the government reinstated the ban on 344 FDC medications, which included these 14 pharmaceuticals, it was originally announced on March 10, 2016.
At the Delhi High Court, more than 30 pharmaceutical firms, including Pfizer, Alkem Laboratories, Glenmark, Procter and Gamble (P&G), and Cipla, contested the prohibition.
The prohibition was overturned by the HC on December 1, 2016, and the government then appealed to the Supreme Court.
The Supreme Court overturned the High Court's decision and ordered that a board known as the Medications Technical Advisory Board (DTAB) review the medications again.
In accordance with the SC's instructions, an expert committee was established to investigate the medications and deliver a report in April 2022.
The expert committee had advised, in its report presented on April 1, 2022, that "there is no therapeutic justification" for the 14 FDCS outlawed and that these combination medications "may involve risk to human beings."
This recommendation was made in the notice gazette, which was published on June 2.
According to the announcement, in the case of the 14 prohibited combination medications, the expert committee said that,
"It is necessary to prohibit the manufacture, sale or distribution of this FDC under section 26 A of the Drugs and Cosmetics Act, 1940," .
Given the above, it is not reasonable to impose any regulations or restrictions that would prevent usage in patients. Thus, only a ban under Section 26A is advised, according to the notice, which was referencing the suggestions of the expert group.
The authority of the Union Government to control, impede, or forbid the manufacturing of a medicine or cosmetic product "in the public interest" is discussed in Section 26A.
According to a recent Health Ministry announcement, the following FDC medications are prohibited in India:
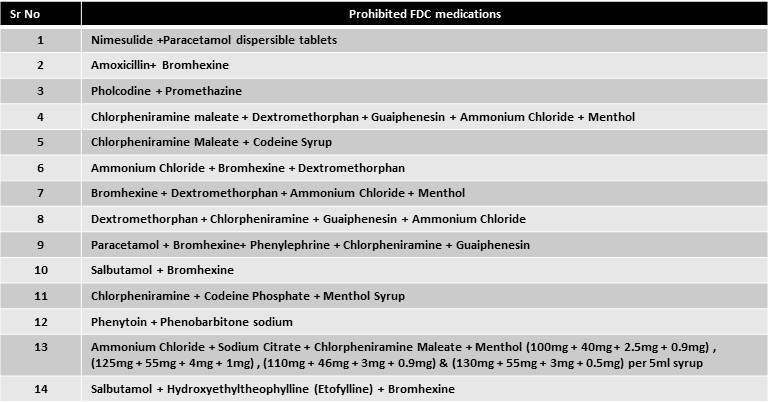
Key messages
-
With immediate effect, 14 fixed-dose combinations (FDC) medications have been outlawed by the Union Health Ministry. The majority of the time, illegal medications were utilised to treat bronchitis, seizures, fever, and cough.
-
The warning states that an expert group concluded that "there is no therapeutic justification for these FDCs, and these drugs may involve risk to humans."
Disclaimer- The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of M3 India.
About the author of this article: Dr Monish Raut is a practising super specialist from New Delhi.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries