Intrahepatic Cholestasis of Pregnancy
M3 India Newsdesk Sep 02, 2024
This article outlines Intrahepatic Cholestasis of Pregnancy (ICP), focusing on symptoms, and management to alleviate maternal pruritus and protect fetal health. It also emphasises the need for timely intervention and follow-up.
Intrahepatic cholestasis of pregnancy (ICP) is characterised by pruritus and an increase in serum bile acid concentrations, mostly developing in the late second and third trimester and rapidly resolving after delivery.
Incidence
In pregnancy, ICP is the most common liver disease [1]. The incidence varies from <1 to 27.6 per cent worldwide [2,3]. In Indian Asian and Pakistani Asian populations, the incidence is 1.2 to 1.5 per cent [4].
Risk factors
- A history of ICP
- Multiple gestations
- Chronic hepatitis C virus infection,
- Personal or family history of intrahepatic cholestasis
- Advanced maternal age [5]
Clinical presentation
-
The first symptom is mild to intolerable pruritus. It generally starts and predominates on the palms and soles and becomes worse at night.
- Steatorrhea, soreness in the right upper quadrant, nausea, and decreased appetite are possible side effects. Symptoms usually develop during the late second or third trimester.
- In cases of in vitro fertilisation, there may be transient first-trimester symptoms due to ovarian hyperstimulation syndrome [6,7].
- If other features of liver failure are present, a search for other causes of liver disease should be done. No primary skin lesions are associated with this disease but scratch marks, excoriations, and prurigo nodules secondary to scratching may be present.
- In 14 to 25 per cent of patients, Jaundice occurs one to four weeks after the onset of itching. Jaundice without pruritus is rare.
Laboratory finding
- In >90 percent of affected pregnancies increase in serum total bile acid concentration is the first and only laboratory abnormality. Pruritus may precede laboratory abnormalities.
- Although some variation in laboratory criteria for the upper limit of the normal reference range for bile acid exists among guidelines [8,9], because of differences in laboratory methods, fasting status, population studied, and gestational age at diagnosis, total serum bile acid cut-off levels reported in the literature vary [10].
- In general, postprandial total serum bile acid levels are greater than those during fasting.[11,12].
- Measuring total serum bile acid levels in the non-fasting state may improve diagnostic accuracy and assessment of disease severity [12,13].
- In 60 percent of cases Serum aminotransferases are increased, usually less than two times the upper limit of normal.
- Alkaline phosphatase is increased, but non-specific for cholestasis during pregnancy due to expression of the placental iso-enzyme.
- Total and direct bilirubin concentrations are increased in 25 per cent of cases and rarely exceed 6 mg/dL.
- gamma-glutamyl transpeptidase (GGT) is usually normal but modestly elevated in 30 per cent of cases.in most other forms of cholestatic liver disease GGT levels increase parallel to other cholestatic markers.
- The prothrombin time is usually normal. It may be prolonged due to vitamin K deficiency from fat malabsorption due to severe steatorrhea or secondary to the use of bile acid sequestrants (cholestyramine).
- ICP is not associated with abnormalities on imaging (biliary ducts are not dilated, hepatic parenchyma appears normal).
Pathology
There is cholestasis without inflammation. Zone 3 is dominated by bile plugs in hepatocytes and canaliculi. The portal tracts are unaffected in IHCP. Maternal bile acids cross the placenta.
Transplacental gradients facilitate fetal clearance of bile acids in normal pregnancies but are reversed in cholestatic pregnancies, which causes the accumulation of bile acids in the fetus and amniotic fluid and carries significant risk for the fetus.
Pathophysiology of adverse pregnancy outcome
The pathophysiology of pregnancy morbidity and fetal death in ICP are not well understood.
- Due to high levels of bile acids, sudden development of a fetal arrhythmia [14] or vasospasm of the placental chorionic surface vessels. Coexistent pregnancy complications (eg, gestational diabetes, preeclampsia) may also add to the pathology. [15].
- Bile acids increase the expression of myometrial oxytocin receptors, which may lead to an increase in preterm labour and spontaneous preterm birth. Cases with spontaneous preterm birth appear to have an earlier onset of pruritus.
- Elevated total bile acids may be related to low birth weight [16-19]; however, fetal growth restriction and oligohydramnios are not features of the IHCP. In particular, the risk of stillbirth increased with serum total bile acid levels ≥100 micromol/L. The risk of stillbirth also increased with increasing gestational age, particularly beyond 34 to 36 weeks.
Management
The management of ICP has two main goals:
- Reducing bothersome symptoms
- Reducing the risk of perinatal morbidity and mortality
While pruritus might be annoying, ICP is not linked to any other severe maternal aftereffects.
Ursodeoxycholic acid (UDCA) is the preferred treatment of maternal pruritus due to ICP, with a dose of 300 mg three times a day (or 15 mg/kg per day) until delivery, but 300 mg twice daily (or 10 mg/kg per day) is also reasonable [8].
The maximum dose is 21 mg/kg per day. Mild nausea and dizziness have been noted in patients. There is a decrease in pruritus within one to two weeks, and biochemical improvement in three to four weeks after starting the treatment.
Refractory cases
If pruritus remains intolerable after the maximum dose of UDCA, one of the following drugs can be added [20].
-
S-adenosyl-methionine
- Cholestyramine
- Rifampin (also known as rifampicin)
For patients who are unable to take UDCA, Alternative drugs may be considered but have less efficacy.
- Take 25 milligrams of hydroxyzine orally every 6 to 8 hours.
- Chlorpheniramine 4 mg orally every four to six hours.
- Calamine lotion or aqueous cream with 2 per cent menthol may also relieve pruritus.
- Dexamethasone 12 mg orally per day.
Evidence of efficacy
UDCA have modest maternal effects, but no significant fetal or newborn benefits.
Antepartum fetal assessment
All pregnancies with ICP should be followed with twice weekly modified biophysical profiles, but antepartum fetal testing to identify fetuses at risk of demise in the setting of ICP is unproven.
Timing of delivery
The Royal College of Obstetricians and Gynecologists (RCOG) guideline on ICP recommends [21]
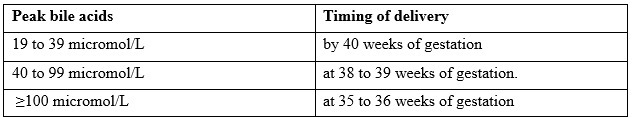
The Society for Maternal-Fetal Medicine (SMFM) and the American College of Obstetricians and Gynecologists (ACOG) advise [22]:

Delivery
Continuous fetal monitoring during labour is indicated.
Postpartum course
- The risk for postpartum haemorrhage is not increased when ICP is managed with UDCA [23]
- Pruritus usually disappears in the first few days postpartum, accompanied by normalisation of serum bile acid concentrations and other liver tests.
- ICP is not a contraindication to breastfeeding.
Follow-up
- Liver biochemical tests and bile acid concentration should be checked if the patient remains symptomatic and the patient should be referred to a hepatologist.
- The Centers for Disease Control and Prevention consider estrogen-progestin contraception an acceptable choice for individuals with a past history of ICP since the benefits generally outweigh the risks [24].
- However, in patients with cholestasis related to past use of estrogen-progestin contraceptives, non-estrogen methods of contraception are preferred due to the increased risk for recurrent cholestasis.
Case scenario
A 34 yrs. old G5P2L2A2 with 34 weeks 2 days pregnancy with gestational diabetes on insulin presented to OPD with complaints of itching all over the body for the last two weeks.
The itching started first on the palm and soles which gradually increased and it was worse in the night. On examination, there were scratch marks on the legs and arms. Icterus is not present and all other antenatal examinations were within normal limits.
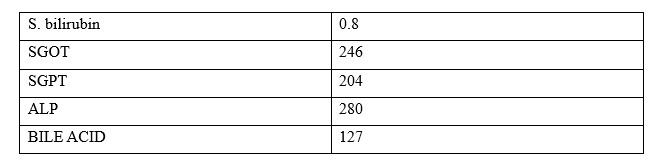
UDCA 300 mg thrice a day started. Fetal surveillance was done twice weekly and induction of labor was planned at 35 completed weeks.
The patient was induced with prostaglandin E2 gel and delivered a 3.5 kg female baby. Postpartum was uneventful. The patient was relieved of symptoms 3 weeks postpartum and was counselled for bilateral tubal ligation after six weeks.
Disclaimer- The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of M3 India.
About the author of this article: Dr Supriya Chaubey is an Assistant professor at HIMSR, New Delhi.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries