Optimising Influenza Treatment: Antiviral Medication Strategies for Effective Management
M3 India Newsdesk Jun 30, 2023
Influenza is a highly contagious respiratory illness caused by the influenza virus. Discover the recommended antiviral medications, including a convenient option, and the intriguing choice between baloxavir and Tamiflu for effective treatment.
Influenza
One of the most widespread infectious illnesses, influenza is an airborne sickness that spreads via periodic epidemics and is extremely contagious. It appears as an acute febrile illness with a range of systemic symptoms, from moderate tiredness to respiratory failure and death. Influenza causes a lot of sick days, distress, and fatalities.
There is significant global morbidity and death due to influenza, but there are few accurate burden estimates from developing countries, including India. Because influenza is sometimes not reported as the primary cause of death on death certificates, estimating influenza mortality is difficult. As a result, estimates of influenza-associated mortality are frequently made using indirect estimation techniques and statistical modelling. According to the most recent WHO statistics, 392,425 fatalities from influenza and pneumonia—or 4.63% of all deaths—occurred in India in 2020. India is ranked #77 in the world by age-adjusted Death Rate, which is 35.30 per 100,000 of the population. Infant mortality and geriatric mortality were the greatest.
The basic goal of treating the flu is to minimise symptoms while your body fights the illness. Since a virus, not a bacterium, is to blame for the flu, antibiotics are ineffective in treating it. To address any secondary bacterial infections that may be present, physicians sometimes may prescribe antibiotics.
Influenza antiviral drugs: Clinicians' guide
A Health Alert Network (HAN), published by the CDC in 2022, offers recommendations for ordering flu antivirals in order of priority for treating flu. Antiviral drugs are a crucial complement to the flu shot in the fight against influenza.
Prescription antiviral medications for influenza may be used to treat the illness and in some cases, prevent it.
For usage during the 2022–2023 influenza season are four antiviral drugs that have been licensed by the U.S. Food and Drug Administration (FDA).
- Oral oseltamivir phosphate (marketed as Tamiflu® or as a generic), inhaled zanamivir (marketed as Relenza®) and intravenous peramivir (marketed as Rapivab®) are three drugs that block the viral neuraminidase enzyme and have activity against both influenza A and B viruses. These drugs are chemically related and act against both influenza A and B viruses.
- The fourth medication is oral baloxavir marboxil (brand name: Xofluza®) which works differently from neuraminidase inhibitors but is effective against both influenza A and B viruses. A cap-dependent endonuclease inhibitor called baloxavir prevents the reproduction of viruses by preventing the transcription of viral RNA. The antiviral medicines amantadine and rimantadine target the M2 ion channel protein of influenza A viruses and are categorised as adamantanes. Because of this, these drugs only work against influenza A viruses and not influenza B viruses. As in prior seasons, the influenza A(H3N2) and influenza A(H1N1) 2009 H1N1 viruses that are now circulating exhibit significant levels of adamantanes resistance (>99%). Therefore, it is not advised to use amantadine or rimantadine to treat or prevent the current strains of influenza A viruses. There is now relatively little antiviral resistance and decreased susceptibility to neuraminidase inhibitors and baloxavir among influenza viruses in circulation, but this might change.
- Early antiviral therapy may minimise the length of fever and other symptoms of an illness, and it may also lower the risk of certain influenza-related complications, such as pneumonia and respiratory failure in young children and otitis media in adults.
- Oseltamivir medication given early to hospitalised adult influenza patients has been shown to decrease mortality in several observational studies.
- Early antiviral therapy with oseltamivir has been shown in observational studies to reduce the length of hospitalisation in pediatric patients.
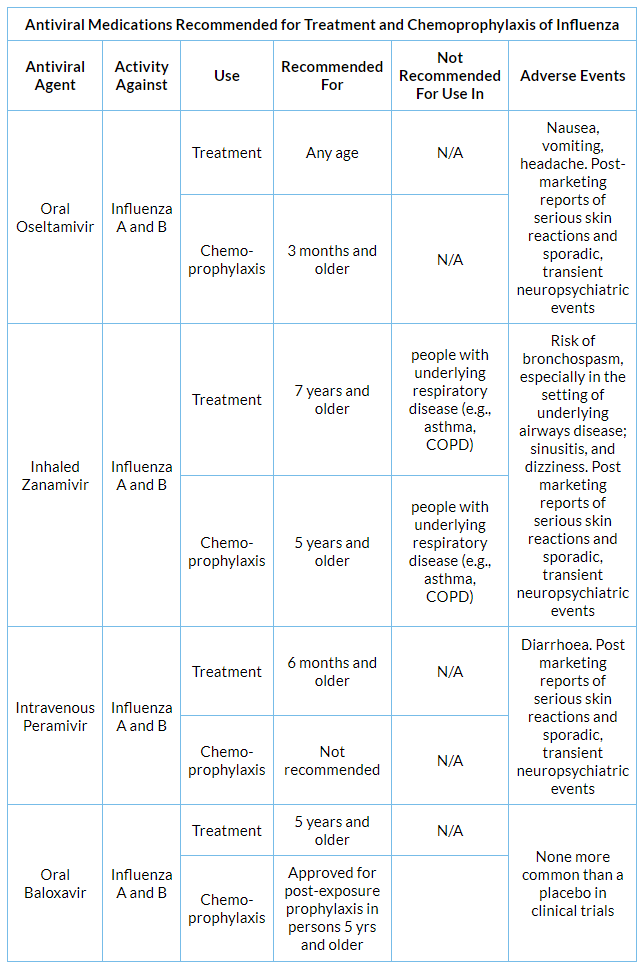
CDC advice on antiviral treatment for influenza
Any patient with confirmed or suspected influenza who is hospitalised has a severe, complex, or progressing illness, or is more susceptible to influenza complications should start antiviral therapy as soon as feasible.
Note: Oral oseltamivir is the antiviral of choice for patients with severe, complicated, or progressive illness who are not hospitalised, as well as influenza patients who are hospitalised.
Any previously healthy, symptomatic outpatient who is diagnosed with confirmed or suspected influenza and who is not at high risk for influenza complications may also be considered for antiviral therapy if treatment can be started within 48 hours of illness onset.
Guide for evaluating influenza diagnostics and treatment when influenza viruses are circulating in the community (regardless of previous influenza vaccination).
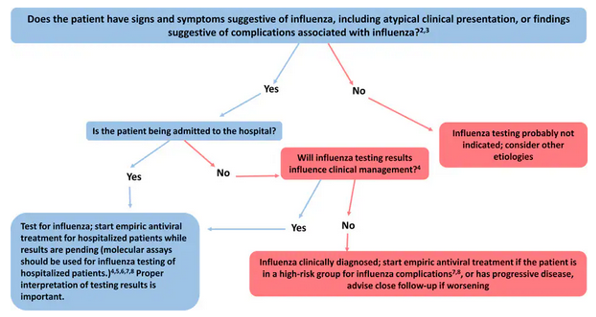
- When choosing an antiviral medication for outpatients at a higher risk of developing a serious illness, clinical judgment is crucial. This judgment should be based on the patient's disease severity and progression, age, underlying medical problems, the possibility of influenza, and time since the beginning of symptoms.
- Antiviral therapy should begin as soon as feasible following the commencement of the disease, preferably within 48 hours after the onset of symptoms, where needed. However, when begun after 48 hours of sickness onset, antiviral medication may have some advantages for patients with severe, complex, or progressive illnesses as well as for those who are hospitalised.
Antiviral therapy decisions should not be delayed for test confirmation of influenza
- While influenza vaccination is the best way to prevent influenza illness, a history of influenza vaccination does not rule out the possibility of influenza virus infection in an ill patient with clinical signs and symptoms compatible with influenza.
- If therapy can be started within 48 hours of the commencement of the illness, antiviral medication may also be considered for any previously healthy, symptomatic outpatient with confirmed or suspected influenza who is not at higher risk of severe disease. Numerous randomised controlled clinical trials (RCTs) and meta-analyses of RCTs have shown the effectiveness of early treatment with neuraminidase inhibitors (begun within 48 hours of illness onset) in reducing the duration of fever and illness symptoms in otherwise healthy children and adults with uncomplicated influenza compared to placebo.
- Treatment options for outpatients with acute, uncomplicated influenza include oral oseltamivir, inhaled zanamivir, intravenous peramivir, and oral baloxavir.
- For uncomplicated influenza, it is advised to take two doses of oral oseltamivir or inhaled zanamivir every day for five days, or one dose of intravenous peramivir or oral baloxavir for one day.
- Data on baloxavir's effectiveness or safety during pregnancy, as well as information on its presence in human milk, effects on breastfed children, and effects on milk supply, are all lacking, hence not recommended.
- For the treatment of pregnant women, oral oseltamivir is preferable. It is advised that pregnant persons take the same dosage of antiviral medication as non-pregnant people.
- For patients with severe or complicated illness with suspected or confirmed influenza (e.g., pneumonia, or exacerbation of underlying chronic medical condition) who are not hospitalised, antiviral treatment with oral or enterically-administered oseltamivir is advised as soon as possible. In individuals with severe influenza illness, there is inadequate evidence for both intravenous peramivir and inhaled zanamivir. Baloxavir therapy for non-hospitalized individuals with severe influenza illness lacks evidence from clinical studies.
Case example
A 32-year-old healthy IT professional comes to you with a 24-hour history of 101°F fever, coughing, and runny nose. She tests positive for influenza B because you decide to test her since you are seeing a few random flu cases in your workplace.
You inform her that while she does not fall into the high-risk group, you might nevertheless recommend antiviral medication to decrease the duration of her sickness. She wishes to be treated. You have four options.
- In OPD, IV peramivir (Rapivib) is impracticable and does not provide any further advantages.
- Another choice is zanamivir (Relenza), a powder that is breathed. As opposed to an MDI, it employs a Diskhaler, which is less prone to inappropriate inhalation. Its usage is restricted to 2 sizable, high-risk groups since it is not licensed for use in people with COPD or asthma. Since there is no generic alternative, the price will probably be a little high. Most patients would rather take a tablet than inhale a powder that could be moderately unpleasant.
- Since generic oseltamivir (Tamiflu) is readily accessible, it is most likely the best choice for those without pharmacy insurance. With your younger pediatric patients, having the choice of pill or suspension is helpful.
- The newest influenza antiviral medication is baloxavir, and it is the fourth choice. Baloxavir is only permitted for anyone above the age of 12 but oseltamivir may be taken at any age. Oseltamivir is administered in 10 doses over the course of 5 days; baloxavir is administered once.
If you had to choose one medicine, would it be baloxavir or generic Tamiflu?
While the two antivirals are equally efficacious, baloxavir is more convenient to administer. By taking Tamiflu with meals, the GI side effects may be lessened.
Disclaimer- The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of M3 India.
About the author of this article: Dr Monish Raut is a practising super specialist from New Delhi.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries