Guideline updates & notable drug approvals in Rheumatology from 2019: Dr. Rohini Handa
M3 India Newsdesk Dec 31, 2019
The year 2019 has been an exciting one for Rheumatology as the gap between the bench and bedside narrows slowly but surely! Here, Dr. Rohini Handa reviews the major disease classification criteria updates, notable drug approvals, and the recent focus on immune checkpoint inhibitor related rheumatic diseases.

Major updates in Rheumatology in 2019:
- New classification criteria for systemic lupus erythematosus (SLE) and IgG4-related disease (IgG4 RD) were unveiled
- Updated recommendations for ankylosing spondylitis (AS), SLE, Sjogren’s syndrome and antiphospholipid syndrome (APS) among others were released
- New drug approvals included Upadacitinib for rheumatoid arthritis (RA) and Nintedanib for scleroderma-related interstitial lung disease
- The field of osteoporosis saw the launch of Romosozumab
- Emerging syndromes in sharp focus were the immune checkpoint related rheumatic diseases
EULAR/ACR classification criteria for Systemic Lupus Erythematosus (SLE)
The 2019, the European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) classification criteria for SLE were developed to strike a balance between specificity and sensitivity. [1]
The obligatory entry criterion is a positive ANA at a titre of ≥1:80 on HEp-2 cells or an equivalent positive test at least once. This is followed by additive weighted criteria grouped in 7 clinical (constitutional, haematologic, neuropsychiatric, mucocutaneous, serosal, musculoskeletal, renal) and 3 immunologic (antiphospholipid antibodies, complement proteins, SLE‐specific antibodies) domains, and weighted from 2 to 10. Patients accumulating ≥10 points are classified as SLE.
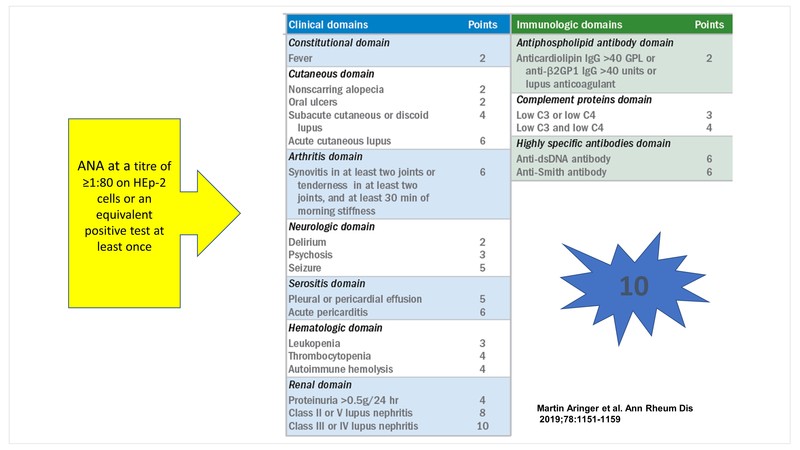
Some caveats that clinicians need to keep in mind are:
- At least one clinical criterion is required
- A criterion cannot be counted if an explanation other than SLE is more likely
- Occurrence of a criterion on at least one occasion is sufficient
- Criteria need not occur simultaneously
- Within each domain, only the highest weighted criterion is counted toward the total score
The sensitivity and specificity of these criteria are 96.1% and 93.4% respectively representing an increased specificity compared to the 2012 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria.
| 2012 SLICC criteria | 2019 EULAR/ACR criteria | |
| Sensitivity | 96.7% | 96.1% |
| Specificity | 83.7% | 93.4% |
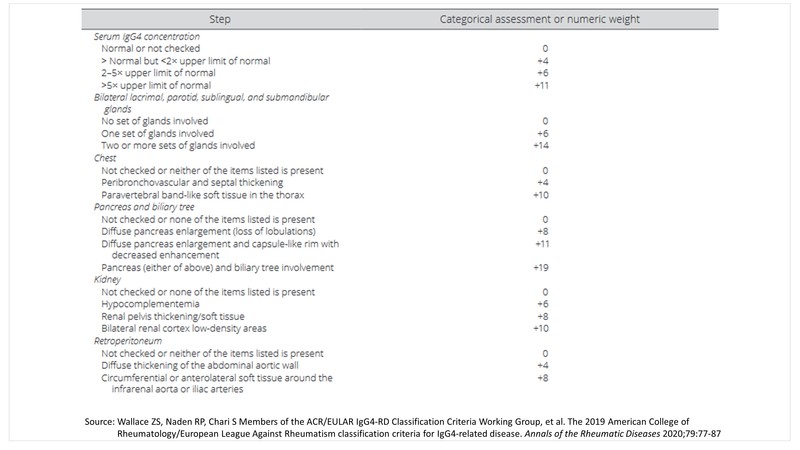
ACR/EULAR classification criteria for IgG4-related disease (IgG4-RD)
The last year also saw the launch of the 2019 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria for IgG4-related Disease. [2]
The panel came up with three steps to classifying a patient with IgG4-RD:
Step 1: Identify at least one organ involvement from the list of 10 organs to typify the disease- pancreas, bile ducts, orbits, major salivary glands, retroperitoneum, lacrimal glands, pachymeninges, kidneys, aorta, thyroid gland.
Step 2: Rule out at least one exclusion from the list of exclusions provided by the panel- 21 in total which include clinical, serological, radiology, and pathology exclusions.
Step 3: Identify individual disease pointers under the list of 8 inclusion criteria domains framed by the panel, the point total of which should add up to a score of ≥20.
Step 4: Classification for IgG4-RD can be made if steps 1, 2, and 3 are met.
2019 Update for Ankylosing Spondylitis (AS) and Non-radiographic Axial Spondyloarthritis (SpA)
The 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis incorporated the latest evidence in the field of spondyloarthritides. [3]
According to these recommendations AS and non-radiographic axial SpA are treated in a similar fashion. TNFi, Secukinumab, and Ixekizumab are favoured over Tofacitinib.
- TNF inhibitors are recommended over IL-17 blockers as the first biologic to be used based on greater experience with TNFi and familiarity with their long-term safety and toxicity.
- Secukinumab or Ixekizumab is recommended over the use of a second TNFi in patients with primary non-response to the first TNFi.
- Co-administration of low-dose Methotrexate with TNFi is not recommended.
- Sulfasalazine is recommended only for persistent peripheral arthritis when TNFi are contraindicated.
- For patients with unclear disease activity, spine or pelvis magnetic resonance imaging could aid assessment.
- Routine monitoring of radiographic changes with serial spine radiographs is not recommended.
EULAR update for Systemic Lupus Erythematosus (SLE), Sjogren’s syndrome, Antiphospholipid Syndrome (APS)
EULAR too released a series of updated treatment recommendations pertaining to SLE, Sjogren’s syndrome and APS. [4]
- The lupus guidelines re-emphasised the importance of hydroxychloroquine which is the anchor drug for all patients with SLE unless some contraindications exist. The dose of corticosteroids should be kept to the bare minimum dose that keeps the disease activity in control. Immunosuppressive drugs such as Methotrexate, Azathioprine and Mycophenolate are recommended as steroid sparing agents.
- The EULAR APS guidelines and indeed all recent publications recommend against the use of Rivaroxaban in patients with APS.
Treatment of Rheumatoid Arthritis (RA)
Upadacitinib (Rinvoq), a JAK 1 inhibitor (15 mg p.o. once daily) was approved by US FDA on 16 August 2019 for the treatment of adults with moderately to severely active RA who have had an inadequate response or intolerance to Methotrexate. This drug was evaluated in a series of robust, well designed phase 3 trials: SELECT-COMPARE, SELECT-NEXT, SELECT-EARLY, SELECT-MONOTHERAPY, SELECT-BEYOND, SELECT-SUNRISE and SELECT-CHOICE.
It is interesting that SELECT-COMPARE, a multicentre, randomised double-blind study demonstrated that Upadacitinib, 15 mg, once daily, was superior to placebo or Adalimumab on background Methotrexate (MTX) for treating signs and symptoms of RA. This drug also inhibited radiographic progression versus placebo at 26 weeks.
Drug approval for Scleroderma
The year 2019 also brought a glimmer of hope to the otherwise barren therapeutic landscape of scleroderma. Nintedanib, first approved in 2014 for adult patients with idiopathic pulmonary fibrosis (IPF), got approval for SSc-ILD in September 2019. The pivotal trial SENSCIS was a Phase III double-blind randomised, placebo-controlled trial including 576 patients from 194 trial sites across 32 countries. The primary endpoint was annual rate of decline in FVC in patients with SSc-ILD.
This trial published in NEJM on 20 May 2019 demonstrated that Nintedanib slowed the loss of pulmonary function by 44% (41 mL/year) in patients with SSc-ILD relative to placebo, as measured by FVC over 52 weeks. Adverse reactions include diarrhoea, nausea, vomiting, skin ulcers, transaminitis, and hypertension.
Drug approval for Osteoporosis
The other notable drug approval of 2019 was Romosozumab, a sclerostin inhibitor with dual mode of action- increased bone formation as well as reduced bone resorption. It is recommended as a 210 mg subcutaneous injection once a month for 12 months. However, it may increase the risk of myocardial infarction, stroke, and cardiovascular death and should not be initiated in patients who have had a myocardial infarction or stroke within the preceding year. Romosozumab received European Union approval in December 2019.
Immune checkpoint inhibitor-related rheumatic diseases
The disease entities in limelight in 2019 were the immune checkpoint inhibitor related rheumatic diseases. The growing use of checkpoint inhibitors (CPIs) in Oncology has spawned novel immune-related adverse events (irAEs) that can affect any organ system including the musculoskeletal system.
These are different from the traditional infections seen with immunosuppression and comprise autoimmune/autoinflammatory conditions triggered by treatment. CPIs target two major signalling pathways: cytotoxic T lymphocyte-associated antigen 4 (CTLA4) with CD28 or programmed cell death protein 1 (PD-1) with programmed cell death ligand 1 (PD-L1). CPIs enhance the self-immune response against tumour cells.
The irAEs include arthralgias/arthritis, myalgias/myositis, polymyalgia rheumatica, SLE, RA, Sicca syndrome etc. The irAEs may persist despite stoppage of CPIs. Treatment of irAEs entails use of corticosteroids and sometimes conventional synthetic or biologic disease-modifying drugs.
Mobile apps & big data
A very interesting set of recommendations dealt with mobile apps aiding self-management in people living with rheumatic and musculoskeletal diseases [5] acknowledging the fact that mobile apps are an integral part of modern day living and rheumatic diseases are no exception.
EULAR also took the initiative of providing a framework for big data in rheumatic musculoskeletal diseases. [6] Big data refers to humongous volumes of data gathered from a variety of diverse sources like electronic health records, social media and health apps, insurance databases, clinical trial repositories, sites recording spontaneous adverse reaction reports, etc. Big data is characterised by 5 Vs:
- Volume of data
- Variety of data
- Velocity of processing
- Veracity
- Value of data
This needs special computational tools to handle data in the realm of not gigabytes (109) but terabytes (1012 bytes), petabytes (1015 bytes) or zettabytes (1021 bytes). The exact potential is yet to be uncovered.
Click here to see references
Disclaimer- The views and opinions expressed in this article are those of the author's and do not necessarily reflect the official policy or position of M3 India.
The author, Dr. Rohini Handa is a Senior Consultant Rheumatologist from Delhi.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries