Chronic Kidney Disease-Mineral Bone Disorder (CKD-MBD): A primer by Dr. Alan Almeida
M3 India Newsdesk Nov 07, 2019
Dr. Alan Almeida discusses chronic kidney disease-mineral bone disorder (CKD-MBD), focusing on abnormal calcium and phosphorus metabolism, associated biochemical changes, bone disease, and vascular calcifications.

The term ‘renal osteodystrophy’ which described mineral and hormone disturbances caused by renal damage, has now been replaced by the term, ‘chronic kidney disease-mineral bone disorder (CKD-MBD)’. CKD-MBD represents a systemic disorder of mineral and bone metabolism that affects not just the bone but also heart and blood vessels.
CKD-MBD is thus characterised by the following:
- An abnormal metabolism of calcium, phosphorus, parathyroid hormone (PTH) or vitamin D
- Bone abnormalities: volume, linear growth, mineralisation, strength or turn over
- Calcification of vessels and soft tissue
The kidneys maintain calcium and phosphorous homeostasis. A complex interplay exists between the essential calcium and phosphorus with hormones such as parathyroid hormone (PTH), calcitriol, and fibroblast growth factor 23 (FGF-23). Calcium and phosphorous have a role to play in cell signaling and energy and contribute to skeletal structure and strength.
There is a disorder of calcium and phosphorus homeostasis in CKD. The disordered mineral metabolism, observed early in the course of CKD, is characterised by high circulating levels of PTH and FGF28, and reduction in circulating calcitriol levels. The effects of this disordered biochemical environment is manifest with altered bone structure (bone pathology) associated with bone pain/fractures, and vascular calcification which is associated with cardiovascular disease morbidity and mortality.
Phosphorus metabolism
A major portion of phosphorus is in the bone. The levels of phosphorus in the body depends on the interaction between the intestines, kidney, and bone, mediated by calcitriol, PTH, and FGF-23. Total serum phosphorus (organic and inorganic) amounts to 11-12mg/dl. However, inorganic phosphate (2.5 - 4.5 mg/dL) is generally used in clinical medicine.
Small intestinal (duodenum and jejunum) absorption is both active and passive. Calcitriol acts on the sodium-phosphate co-transporter 2b (Npt2b) on the intestinal epithelial brush border increasing phosphorous. Foecal and urinary excretion of excess phosphorus takes place. Phosphate handling by the kidneys includes reabsorption of the filtered phosphorous at both the proximal tubule (70-80%) or in the distal tubule (20-30%) via Npt2a and Npt2c. On the other hand, PTH and FGF-23 stimulate phosphorus excretion by the kidney.
Calcium metabolism
Normal serum calcium levels are maintained between 8.5 and 10.5 mg/dL. The interaction of the intestine, kidney and bone determines calcium levels (majorly resident in the bone). Total serum calcium is ionised (48%) and the remainder is either protein-bound or complexed with anions (e.g., phosphate, bicarbonate). The ionised component is physiologically active.
Calcitriol is important for intestinal calcium absorption (20-25% of dietary calcium) via the TRPV5 and TRPV6 (calcitriol induction) transporters and paracellular pathways. Just as with phosphorous, renal handling involves filtration followed by major passive reabsorption of the filtered calcium by the proximal tubule and a distal tubule reabsorption of 5-10% (active regulation of TRPV5 and TRPV6.
PTH is the most important hormone in the maintenance of serum calcium concentrations. Calcium sensing receptors (CaSR) located on the parathyroid glands, respond to small changes in serum ionised calcium concentrations and acts to stimulate or inhibit PTH release. PTH raises serum calcium by stimulating bone resorption, promoting intestinal absorption of calcium by increasing circulating calcitriol levels and enhancing tubular reabsorption of calcium.
What happens when the kidney is diseased?
Phosphorous excretion impairment accompanies the decline in glomerular filtration rate associated with CKD progression. FGF-23 (phosphaturic hormone) and PTH levels rise to increase phosphorus excretion in the surviving nephrons. Elevated FGF-23 levels inhibit calcitriol (active vitamin D), which not only retards intestinal absorption of phosphorus but also calcium (resultant increase in PTH levels). Normalcy of serum calcium and phosphorus are maintained at the expense of increased circulating levels of FGF-23 and PTH (often referred to as the "trade-off hypothesis”).
What are the biochemical changes associated with CKD-MBD?
Fibroblast Growth Factor 23 (FGF-23)
FGF 23 and its co-receptor µ-klothos are the main regulators of phosphate homeostasis. At the level of the kidney, down-regulation of phosphorus transporters results in reduction of phosphorous reabsorption and resultant increased phosphorus excretion (‘phosphaturic’). Inhibition of conversion of vitamin D (1-a hydroxylase) to its active form, decreases intestinal phosphorous (and calcium) reabsorption.
FGF-23 decreases PTH levels and increases expression of CaSR and Vitamin D receptors (VDR). Circulating levels of FGF-23 rise very early in the course of CKD, even before there are increases in serum levels of phosphorus and PTH, and progressively rises as kidney function declines in CKD. FGF-23 inhibits PTH in healthy individuals but not in CKD patients.
Elevated FGF 23 levels in pre-dialysis CKD, have serious implications being associated with kidney disease progression, cardiovascular disease events and all-cause mortality. A similar strong association between higher FGF-23 levels and cardiovascular disease events and all-cause mortality has been noted in dialysis patients.
Parathyroid hormone
PTH, by binding to the PTH 1 receptor in the bone and kidney maintains calcium homeostasis. Bone resorption releases calcium and phosphorus into the circulation. By upregulating calcium transporters in the kidney, increased calcium reabsorption in the distal tubule also promotes phosphorous excretion by promoting catabolism of its transporter. In the kidneys, PTH by increasing the activity of 1-a hydroxylase, activates vitamin D (calcitriol) and indirectly increases intestinal reabsorption of calcium (and phosphorus).
Ionised calcium concentrations detected by the calcium sensing receptors at the parathyroid gland permit minute-to-minute variation in PTH release. Low ionised calcium increases and elevated ionised calcium inhibits release. PTH release also occurs in response to hyperphosphatemia and low circulating calcitriol levels.
Secondary hyperparathyroidism (elevated PTH levels) occurs in CKD. PTH levels rise when the GFR falls below 60 ml/min/1.73 m2. With progressive CKD, PTH levels rise, as is noted with FGF-23. Hypocalcaemia, low calcitriol levels, hyperphosphatemia and skeletal resistance to PTH are responsible for the development of secondary hyperparathyroidism in CKD. Elevated PTH, a uremic toxin, causes cardiac dysfunction, extraskeletal calcification, anaemia, encephalopathy, peripheral neuropathy, and immune dysfunction.
Calcitriol
1,25 dihydroxyvitamin D3, the active metabolite of fat-soluble vitamin (cholecalciferol), is converted in the kidney via the enzyme 1-a hydroxylase. An inactive metabolite, 24,25-dihydroxyvitamin D, is hydroxylated by 24-a hydroxylase. Calcitriol increases circulating concentrations of calcium and phosphorus. Intestinal absorption of calcium and phosphorus, tubular reabsorption of calcium and phosphorous occurs and PTH release from the parathyroid gland is suppressed.
PTH and low phosphorus stimulate production of calcitriol. On the other hand, FGF-23 inhibits 1-a hydroxylase, while stimulating 24-a hydroxylase in the kidney, whereby there is decrease in circulating calcitriol levels. In CKD, loss of renal tubular cells and hyperphosphatemia inhibit 1-a hydroxylase production. Vitamin D deficiency is also linked to cardiovascular diease events.
Renal Osteodystrophy
The term ‘renal osteodystrophy’(ROD) is restricted to bone abnormalities associated with CKD-MBD. Bone quality and strength is altered through hormonal and metabolic disturbances of CKD. There is an increased fracture risk which contributes to higher morbidity and mortality.
Renal osteodystrophy is broadly classified into 4 categories:
- High turnover bone disease (osteitis fibrosa cystica)
- Low turnover bone disease without increased unmineralised bone (Adynamic Bone disease-ABD)
- Low turnover bone disease with increased unmineralised bone (osteomalacia)
- Mixed uremic osteodystrophy
This classification (TMV system) is possible with use of a bone biopsy which assesses bone turnover (T), mineralisation (M), and volume (V). Dual energy X-ray absorptiometry (DXA) is now an accepted tool in CKD-MBD workup.
High turnover bone disease (osteitis fibrosa cystica)
Bone features of high turnover, normal mineralisation and high volume. High turnover bone disease is due to secondary hyperparathyroidism (elevated PTH) along with elevated bone specific alkaline phosphatase. Therapy is aimed at lowering PTH levels.
Adynamic bone disease (ABD)
Bone biopsy features of adynamic bone disease includes low turnover, normal mineralisation and low to normal volume with oversuppression of PTH. This oversuppression could be due to high doses of active Vitamin D, calcium phosphate binders and in dialysis patients (high dialysate calcium concentrations) or skeletal resistance to PTH.
Older and diabetic patients are prone to the development of ABD. Vascular calcification with its accompanying mortality is noted in association with ABD. Increasing PTH levels by stopping Vitamin D or by lowering dialysate concentrations of calcium or omitting calcium phosphate binders helps in the treatment of ABD.
Osteomalacia
Low turnover, abnormal mineralisation, and low to normal volume characterise osteomalacia. Osteomalacia is also noted with aluminium intoxication associated with the use of aluminium containing phosphate binders. Mineralisation is inhibited by aluminium deposited in the bone.
Mixed uremic osteodystrophy
High turnover, abnormal mineralisation, normal volume– a combination of high turnover and increased osteoid. Therapy is directed at factors that impair mineralisation and to correct biochemical abnormalities so as to reduce bone turnover and increase mineralisation.
Vascular calcification
Arterial calcification- intimal and medial,- is a feature of CKD-MBD. Vascular calcification occurs when vascular smooth muscle cells differentiate to osteoblast-like cells and mineralise. The systemic inflammation, elevated phosphorous, lower levels of circulating mineralisation inhibitors that exist in CKD, contribute to the initiation and propagation of the vascular calcification. Coronary artery calcification is prevalent in 50 to 60% of maintenance hemodialysis patients. Kidney transplantation and nocturnal dialysis may be beneficial in stabilising but not reversing the condition.
Clinical manifestations
In high turnover bone disease, non-specific aches and pains in the lower back, hips, legs, aggravated by weight may occur. Acute, localised bone pain may suggest acute arthritis. Periarticular pain suggests periarthritis (deposition of calcium phosphate crystals). Deformities can result from fractures, extraskeletal calcifications, vascular calcification, pruritus and occasionally calciphylaxis. There may be a gradual onset of muscle weakness.
Biochemical investigations: These include levels of serum calcium, phosphorus, vitamin D metabolites and PTH. In stage 4 CKD, serum calcium levels tend to fall with hyperphosphatemia. Hypercalcemia may result with administration of calcium containing antacids or vitamin D metabolites. Levels of alkaline phosphatase offer an approximate index of osteoblast activity in patients with CKD. Hyperparathyroid bone disease is associated with high levels of alkaline phosphatase; measurement of bone-specific alkaline phosphatase especially in conjunction with high PTH. Osteocalcin is another marker which is not superior to alkaline phosphatase.
Radiology features: They include bone erosions, subperiosteal resorption, ‘pepper pot skull’, ‘rugger-jersey’ spine, brown tumours. Looser zones are characteristic of osteomalacia. DEXA is widely used to assess bone density. Bone biopsy and examination of undercalcified bone sections after double tetracycline labelling provide a definitive and quantitative diagnosis of renal osteodystrophy. This, however, is done in a few centers in India.
Therapy for hyperparathyroidism should be initiated early in CKD in stage 3. Postponed treatment could result in significant skeletal deformities or nodular parathyroid hyperplasia. Management revolves around altering the levels of calcium and phosphorus, altering PTH levels.
- Calcium should be corrected because it is a potent stimulus for PTH secretion. Calcium supplements such as calcium carbonate taken between meals with increasing doses helps.
- Vitamin D supplementation should be done if the 25 hyodroxy vitamin D levels are <30 ng/ml.
- Phosphate lowering should be achieved through use of phosphate binders that bind phosphate in the intestine. They include calcium- based binders, polymer-based binders, metal-based binders and iron-based binders. Aluminium-containing phosphate binders are no longer used as that could result in significant aluminium absorption.
- Dietary protein restriction too is necessary. Magnesium binders are used with caution because hypermagnesemia could have serious adverse effects. The serum levels are lowered with possible lowering of PTH levels.
Binding the Vitamin D Receptor increases intestinal calcium absorption and lowers PTH. Calcium and phosphorus levels are increased. The drugs used are calcitriol, paricalcitol, and doxercalciferol. Lowering circulating PTH levels causes decrease in serum calcium and phosphorous. Cinacalcet and etecalcitide bind the calcium sensing receptor (CaSR) on the parathyroid gland and decrease the PTH levels. Over-suppression of PTH could predispose to development of adynamic bone disease (ABD).
Low turnover bone disease – adynamic bone disease- develops from rigorous suppression of the adaptive response (hyperaparthyroidism) related to increased skeletal resistance to PTH and phosphate overload. Fracture incidence and increased cardiovascular calcification and mortality accompanies low turnover rather than high turnover bone disease.
In low turnover bone disease, low iPTH levels (<100-150pg/ml) in stage 5D are noted. Alkaline phosphatase or bone alkaline phosphatase are normal to low. Serm calcium and phosphate can be normal or elevated, depending on the treatment received (phosphate binders, vitamin D and nutritional status).
Hypercalcemia and hyperphosphatemia may be pronounced in ABD because the bone is unable to buffer calcium and phosphate loads by osseous deposition. Conventional bone X-rays or DEXA may be used but do not show typical features. Bone density may be low, normal or high. A very high CV calcification burden on conventional radiographs may raise suspicion of low turnover bone state. ABD is associated with the highest magnitude of vascular calcification in dialysis patients.
Therapy of Adynamic Bone Disease is to avoid PTH overexpression and restore adequate PTH levels without reinstating secondary hyperparathyroidism. Calcimimetics may need to be avoided, along with reduction or withdrawal of vitamin D metabolites, reduction or withdrawal of calcium containing phosphate binders or lowering of dialysate calcium.
Osteoporosis in Chronic Kidney Disease
Abnormal bone and fracture risk is increased in CKD patients. Osteoporosis may be associated with low, normal, or high bone turnover and is characterised by thin and disconnected trabecular and loss of the plate-like structure. Abnormal mineralisation, increased osteoid characterise CKD osteoporosis.
Measurements of bone turnover markers or bone mineral density by DEXA are of no value. If at all, bone mineral density on DEXA does predict fracture risk and can be of use in monitoring therapeutic drug effects or risk assessments. The only reliable method to diagnose osteoporosis is bone biopsy. Peripheral quantitative tomography (pQCT) after validation may become a useful tool in the future.
Anti-osteoporotic medications can be used to treat osteoporosis in CKD 1 to 3. Bisphosphonates, denosumab, raloxifene, and teriparatide appear to be feasible options. In the FREEDOM trial, denosumab demonstrated a significantly decreased fracture risk versus placebo. In later stages of CKD (3-5), no data are available on the safety and efficacy of any of the antiosteoporotic medications.
In CKD patients with ABD, bisphosphonates and denosumab may aggravate osteoclast paralysis. In CKD patients with secondary hyperparathyroidism, these antireosptive agents may upregulate PTH. In CKD 4 and 5, denosumab may cause, hypocalcemia may develop. This effect may be blunted with coadminstration of vitamin D analogues.
Fractures may be a part of CKD-MBD. Given below is an algorithm for fracture risk-screening and initiation of anti-fracture strategies in patients with CKD.
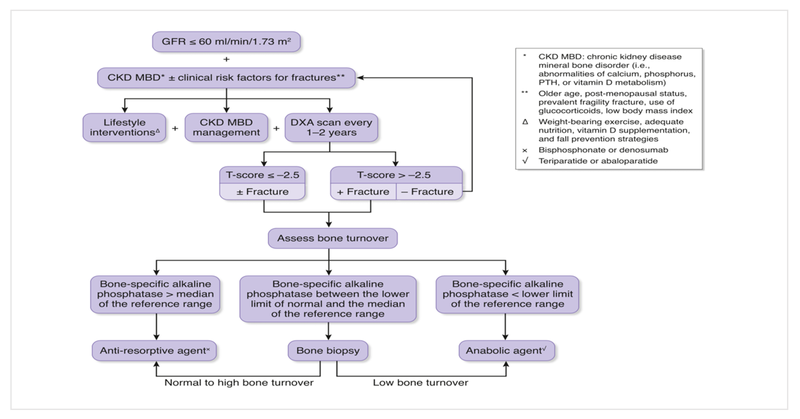
Source: Pascale Khairallah and Thomas L. Nickolas. Management of Osteoporosis in CKD. CJASN June 2018, 13 (6) 962-969; DOI: https://doi.org/10.2215/CJN.11031017
Disturbances of mineral metabolism in CKD lead to serious and debilitating complications unless addressed and treated. The manifestations encompass renal osteodystrophy (skeletal abnormalities) that include osteitis fibrosa, osteomalacia, adynamic bone disease, osteoporosis or at times a mixed renal osteodystrophy. Occasionally, beta2 microglobulin amyloidosis may be a part of the skeletal abnormalities.
Disclaimer- The views and opinions expressed in the article and videos are those of the speakers and do not necessarily reflect the official policy or position of M3 India.
The author, Dr. Alan Almeida is a Consultant Nephrologist and Transplant Physician at P.D. Hinduja Hospital, Mumbai.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries