US FDA approves first test to screen Zika virus in donated blood
IANS Oct 07, 2017
The US Food and Drug Administration (FDA) has approved for the first time a test for detecting the Zika virus in donated blood.
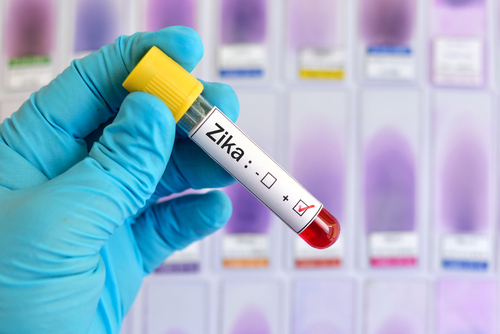
The Zika virus is transmitted primarily by mosquitos (Aedes aegypti), but it can also be spread through blood transfusion and sexual contact. The cobas Zika test, manufactured by Roche Molecular Systems Inc., is intended for use by blood collection establishments to detect Zika virus in blood donations, not for the individual diagnosis of Zika virus infection, the FDA said on Thursday."Today's action represents the first approval of a Zika virus detection test for use with screening the nation's blood supply," said Peter Marks, Director of the FDA's Centre for Biologics Evaluation and Research.
"Screening blood donations for the Zika virus is critical to preventing infected donations from entering the US blood supply," Marks added.The cobas Zika test is qualitative nucleic acid test for the detection of Zika virus RNA in individual plasma specimens obtained from volunteer donors of whole blood and blood components, and from living organ donors. The test's clinical specificity was evaluated by testing individual samples from blood donations at five external laboratory sites, resulting in clinical specificity of more than 99 percent.Although most people infected with Zika virus do not develop symptoms, when symptoms do occur they may include fever, arthralgia (joint pain), maculopapular rash, and conjunctivitis (red, irritated eyes). In addition, Zika virus infection can cause a serious neurological disease in adults, and infection during pregnancy can cause serious birth defects.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries