Torrent Pharma recalls Losartan hypertension tablet in US
IANS Dec 22, 2018
Indian pharmaceutical major Torrent Pharmaceuticals Ltd is voluntarily recalling two lots of Losartan potassium tablets -- used to treat hypertension -- in the US market due to detection of traces of unexpected impurity, the US Food and Drug Administration (USFDA) said.
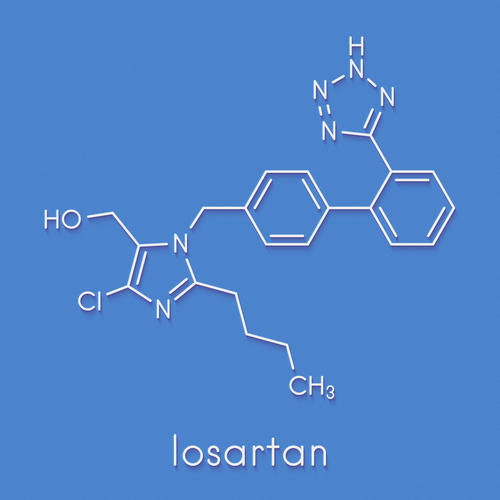
"Torrent Pharmaceuticals Limited is voluntarily recalling two lots of Losartan potassium tablets, USP to the consumer level due to the detection of trace amounts of an unexpected impurity found in an active pharmaceutical ingredient (API) manufactured by Hetero Labs Limited," USFDA said in a statement.
According to USFDA, the impurity detected in the API is N-nitrosodiethylamine (NDEA), which is a substance that occurs naturally in certain foods, drinking water, air pollution, and industrial processes, and has been classified as a probable human carcinogen as per International Agency for Research on Cancer (IARC) classification.
To date, Torrent Pharmaceuticals Ltd has not received any reports of adverse events related to this recall, the statement said. Losartan is used to treat hypertension, hypertensive patients with Left Ventricular Hypertrophy and for the treatment of nephropathy in Type 2 diabetic patients.
Patients who are on Losartan should continue taking their medication as the risk of harm to the patient's health may be higher if the treatment is stopped immediately without any alternative treatment, the statement added. Patients should contact their pharmacist or physician who can advise them about an alternative treatment prior to returning their medication, the USFDA said. The products subject to recall are 100 mg tablets with the expiration date 4/2019 and packed in 30/90/1,000 count bottles, the USFDA said.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries