Strides Pharma Science said on May 21 that it has received approval to conduct clinical trials of antiviral drug Favipiravir, a potential drug for COVID-19 cure.
For our comprehensive coverage and latest updates on COVID-19 click here.
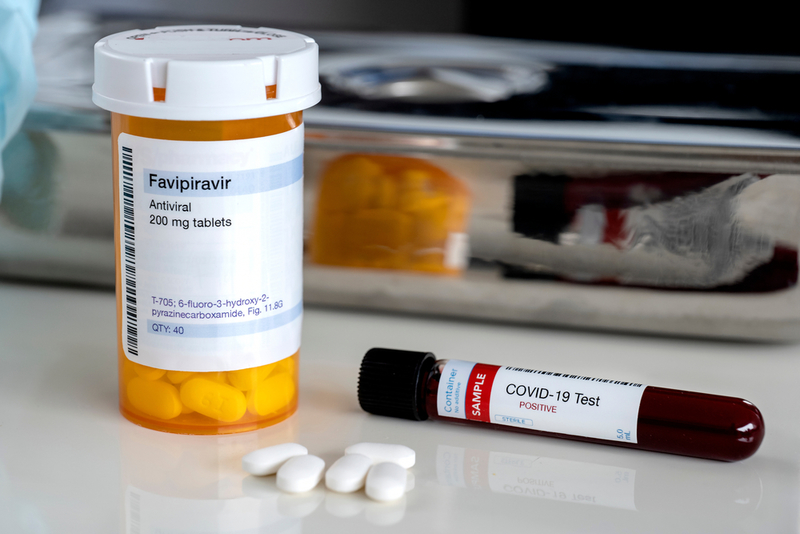
The company has received approval from the Drug Controller General of India (DCGI) to conduct trials of Favipiravir in the country, Strides Founder and Non-Executive Chairman Arun Kumar said. Strides' announcement came after Glenmark Pharmaceuticals said last month that it became the first pharma company in the country to get the nod to conduct Favipiravir trials. Favipiravir is manufactured under the brand name Avigan by a unit of Japan's Fujifilm Holdings Corp.
"We have entered into a new world order with COVID-19, the magnitude and scale of which remains indeterminate. We have maintained our agility and responsiveness to deal with this situation and are pursuing our commitment to 3Ps viz Patients, People and Purpose," R. Ananthanarayanan, CEO & MD, Strides Pharma, said in the announcement of the company's financial results.
"We are determined to play a substantial role in society by bringing affordable and quality healthcare to millions of people around the globe. We strongly believe that our businesses will maintain traction and would grow significantly to culminate into a healthy financial outcome for Strides in FY21," he added.