Polio surveillance enhanced in UP, Maharashtra, Telangana: Health Ministry
PTI Oct 04, 2018
The Health Ministry on October 3 said surveillance has been enhanced in Uttar Pradesh, Maharashtra and Telangana after traces of Polio Virus Type 2 were found in some batches of oral polio vaccine, and asserted the country remains polio-free.
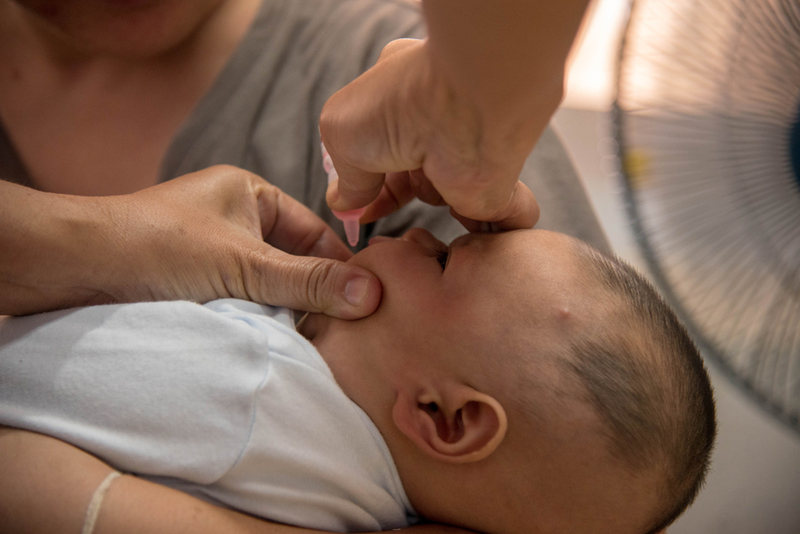
The ministry said a constant vigil is being kept on the shedding of the polio vaccine virus in these areas and it has ordered a probe into the matter. The ministry's remarks came days after traces of Polio Virus Type 2 were found in some batches of oral polio vaccine manufactured by a Ghaziabad-based pharmaceutical company. It further said no sample has tested positive for wild polio virus in sewage or acute flaccid paralysis cases since 2011.
"The country remains polio-free and this status has been maintained for more than seven years since the last wild polio virus case in the country was reported in January 2011. No child has been infected with wild polio virus as reported in some sections of the media," the ministry said in a statement. The government further said that type 2 polio vaccine virus traces, which have been found in bivalent Oral Polio Vaccine (bOPV) vials, is a weakened polio virus and does not cause paralysis and was also earlier used in vaccines till April 2016.
The recipients of such vaccine will usually shed the vaccine virus through fecal route for about 4-6 weeks after which it will die down, the statement said. The Health Ministry has asked the surveillance teams in the three states to track all those children who have been given the vaccine and keep a close watch for any symptoms.
To enhance immunity against type 2 polio virus further, special mop up rounds for administering IPV are being conducted in the specified areas to reach out to such children who may have missed it. This would provide immunity to all the children against all the three types of polio virus including type 2, the statement stated.
After the contamination came to light, the use of all the vaccine supplied by manufacturer was immediately stopped in the country till investigation is completed. "Additional legal samples of bOPV were immediately sent for testing to the Central Drug Laboratory in Kasauli, which confirmed the previous report of presence of traces of P2 polio vaccine virus," the statement said.
The Drugs Controller General of India immediately lodged a case and issued notice to the company asking it to stop manufacturing and supplying till further orders. The MD of the company was immediately arrested, the statement said.
In view of high routine immunization coverage being achieved in India and administration of tOPV till 2016, the risk of any child getting vaccine derived polio disease is practically nil, the statement said. Sufficient polio vaccine from alternate sources is available in the programme to implement Routine Immunization (RI) and Pulse Polio Immunization (PPI) and maintain the immunity against polio-viruses.
The ministry further said the detection of type 2 vaccine virus indicates that a very robust polio surveillance jointly managed by the Ministry of Health and Family Welfare and the WHO is still maintained even after seven years have elapsed since the last wild poliovirus case in the country was reported.
This detection indicated the use of a type 2 polio virus containing vaccine, despite the fact that use of these vaccine had been phased out globally, and in India, in April 2016, as a part of the Polio End Game strategy. The decision to switch to inactivated poliovirus vaccine (IPV) and bivalent Oral Polio Vaccine (bOPV) from trivalent Oral Polio Vaccine (tOPV) in all polio campaigns and routine immunization in India and elsewhere from April 2016 was taken following certification of global eradication of Type 2 wild polio virus.
In India, the last type 2 wild polio virus case was detected in 1999. A team of health ministry, DCGI, ICMR and the WHO is continuously monitoring the situation. "The Government of India is committed as always to ensure that all vaccines used in the programme are safe and effective," the statement stated.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries