Nivolumab shows promising activity in advanced tumours with rare mutations
ESMO Virtual Congress 2020 Sep 23, 2020
Nivolumab monotherapy provided high response and disease control rates in patients with pathogenic exonuclease domain POLE (edPOLE) mutated mismatch repair (MMR)-proficient advanced tumours containing confirmed pathogenic mutations, according to findings presented by Dr. Benoit J.-C. Rousseau of the Memorial Sloan Kettering Cancer Center in New York, USA at ESMO Virtual Congress 2020.
Dr. Rousseau explained that hotspots due to edPOLE mutations generate proofreading defects and hypermutated genomic profiles that may serve as targets for existing therapies.
These hotspots occur rarely in advanced disease, and have not been fully investigated; therefore, the efficacy of nivolumab in patients with MMR-proficient tumours and edPOLE mutation has not been established.
To this end, Dr. Rousseau and colleagues conducted investigation of nivolumab in conjunction with the ACSé Immunotherapy Programme, a nationwide programme launched by the French National Cancer Institute and sponsored by the French network of comprehensive cancer centres (UNICANCER). This programme is investigating the efficacy and tolerance of anti-PD1 agents, such as nivolumab, in multiple phase II single arm cohorts of patients with rare tumours.
Patient selection for this trial was according to mutational functional assessment by a molecular tumour board
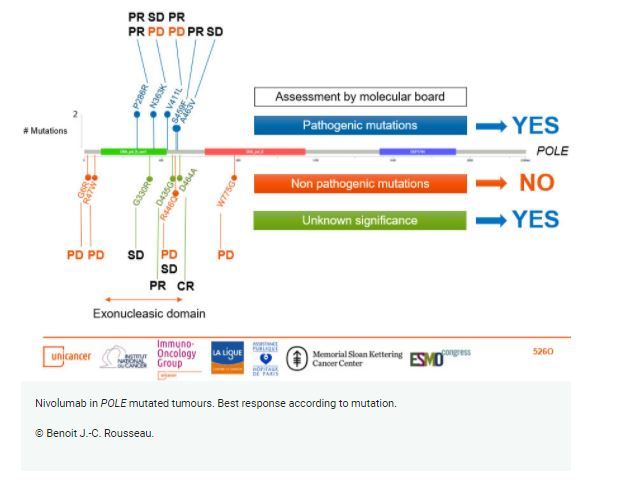
At ESMO 2020, the initial results were presented from the edPOLE cohort participating in the first trial to investigate immunotherapy in patients harbouring missense POLE within or close to the exonucleasic domain. Mutation pathogenicity in patients prospectively selected by an ad hoc molecular tumour board.
All patients in this cohort received intravenous nivolumab at 240 mg every two weeks until disease progression, toxicity, or for up to 2 years in their absence.
The primary endpoint was the objective response rate (ORR) assessed per RECIST v1.1 at 12 weeks.
From January 2018 to April 2020, sixteen patients with a mean age of 57 years were enrolled. Of these, 7 patients had colorectal, 4 endometrial, 2 gastric, and one patient each had pancreatic, biliary and glial tumours. The patients had received a mean of 2.6 prior lines of therapy.
The nivolumab response was greater in patients with pathogenic mutations confirmed by the ad hoc committee.
The ad hoc committee confirmed the pathogenicity of the edPOLE mutation in the tumours of 8 (50%) patients, which included 2 P286R, 2 N363K, 2 V411L, one S459F and one A463V mutations. Three patients had unknown significance mutations and 5 had non-pathogenic mutations.
At 12 weeks, among the 16 patients, the ORR was 38%, which comprised 5 partial responses and 6 stable disease. Five patients experienced progressive disease at this time. These responses were observed exclusively in patients with MMR-proficient colorectal and endometrial cancers. The median follow-up was 7.0 months (min 1.0; max 21.9).
In the group of 8 patients stratified by pathogenic mutations, 4 patients showed response for an ORR of 50%. Stable disease was achieved by 2 patients, which yielded a disease control rate of 75%. Moreover, for the 3 patients with unknown significance mutations, 2 patients had responses and one had stable disease.
In contrast, at 12 weeks, all the 5 patients with non-pathogenic tumours progressed including 2 patients that had died prior to the first evaluation.
When comparing non-pathogenic mutation and pathogenic/unknown mutations groups, the respective median progression-free survival and overall survival were 1.1 vs 5.6 months (hazard ratio 0.19 confidence interval 95% 0.06-0.62) and 5.3 months vs non reached.
The safety evaluation showed that the nivolumab profile was in accordance with safety data reported in other tumour types.
Conclusions
According to the authors, these data represent the first clinical study assessing an anti-PD1 agent in POLE mutated MMR-proficient tumours. They concluded that nivolumab activity appears promising in patients with edPOLE mutated MMR-proficient advanced cancer but may be limited to tumours containing pathogenic mutations.
They emphasised that this result underscored the importance of individual mutational functional assessment by a molecular tumour board. Tumour mutational burden assessment is ongoing.
This UNICANCER study was funded by Bristol Myers Squibb.
This article is a news release from ESMO 2020 Press Meeting. Read the original here.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries