The Central Drug Research Institute (CDRI), Lucknow, has received permission from Drug Controller General of India (DCGI) for carrying out Phase III clinical trials to test the efficacy, safety and tolerability of the antiviral drug Umifenovir. The Phase III activities will be carried out at King George's Medical University (KGMU), Dr Ram Manohar Lohia Institute of Medical Sciences (RMLIMS) and ERA's Lucknow Medical College and Hospital, Lucknow.
For our comprehensive coverage and latest updates on COVID-19 click here.
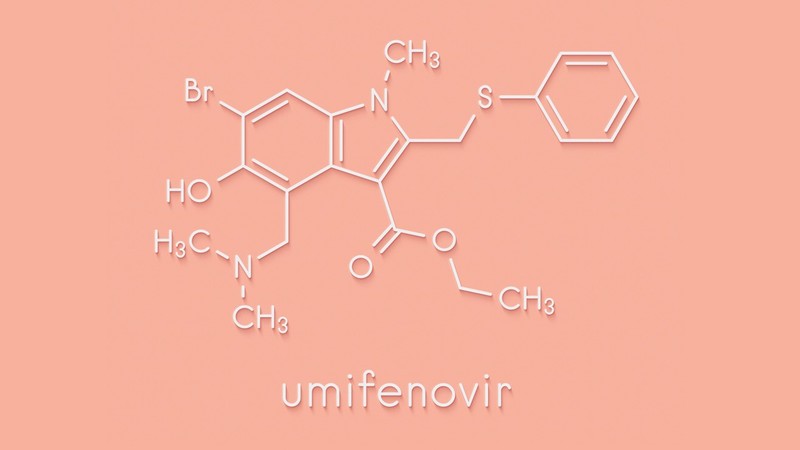
Umifenovir is mainly used for treatment of influenza and is available in China and Russia, with the brand name of Arbidol and has recently come into prominence due to its potential use for COVID-19 patients. To evaluate its efficacy in Indian patients, CDRI has taken up the clinical trial.
These will be randomised, which means the drug would be tested in random manner, double blind, placebo-controlled trials. “This drug has a good safety profile and acts by preventing entry of virus into human cells and also by priming the immune system. Hopefully we will complete the trials in the next two months,” said Dr Ravishankar Ramachandran, Nodal Scientist, CDRI, while speaking with India Science Wire.
